-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Kostolny, Victor Tsang, Johannes Nordmeyer, Carin Van Doorn, Alessandra Frigiola, Sachin Khambadkone, Marc R. de Leval, Philipp Bonhoeffer, Rescue surgery following percutaneous pulmonary valve implantation, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 4, April 2008, Pages 607–612, https://doi.org/10.1016/j.ejcts.2007.12.034
Close - Share Icon Share
Abstract
Objective: Percutaneous pulmonary valve insertion (PPVI) is an evolving alternative to surgical pulmonary valve insertion. The aim of this study is to review the acute complications of PPVI requiring emergency rescue surgery. Patients and methods: Between 09/2000 and 01/2007, 152 patients (pts), received a PPVI. Patient’s charts were reviewed in retrospect. Results: Emergency rescue surgery (ERS) took place in 6 pts (3.9%). Indications for ERS were: homograft rupture two pts, dislodgment of the stented valve in a dilated right ventricular outflow tract two pts, occlusion of the right pulmonary artery one pt and compression of the left main coronary artery one pt. Cardiopulmonary bypass was established through repeat sternotomy incision with femorofemoral cannulation in 2/6 pts. The stented valve was removed in five and replaced with a homograft in three and a valved conduit in two pts. One ruptured homograft was repaired leaving the stented valve in situ. All patients survived, one sustained mild neurological impairment. Conclusion: Although some of the acute complications of PPVI were probably related to a learning curve (4 among the first 50 pts and 2 among the last 102 patients) the need for ERS is unlikely to be completely abolished. This experience highlights the importance of close collaboration between cardiologists and surgeons in these evolving technologies. Highly skilled and responsive surgical back up is necessary to support the introduction and to sustain institutional programmes such as PPVI.
1 Introduction
Classic indications for pulmonary valve replacement are significant pulmonary outflow tract obstruction with or without significant pulmonary insufficiency, RV dilation or RV failure. However, earlier intervention may lead to improvement in exercise tolerance, right ventricular volumes and function [1–3]. Percutaneous pulmonary valve implantation (PPVI) is an emerging alternative to surgical placement of pulmonary valve into the right ventricular outflow tract [4,5]. The prospect of offering a less invasive approach to deal with ventricular outflow tract dysfunction is clearly attractive for the patient, the cardiologist and the surgeon [6]. The aim of this study is to describe acute complications of percutaneous pulmonary valve replacement requiring emergency rescue surgery (ERS). Furthermore, the importance of close interdisciplinary cooperation is highlighted.
2 Patients and methods
Patients presenting for placement of a pulmonary valve into the right ventricular outflow tract (RVOT) at our institution are systematically evaluated regarding their suitability for surgical or interventional approach by a multidisciplinary team.
Current indications for PPVI are RVOT conduits with diameter between 16 and 22 mm, age >5 years and weight >20 kg.
Current limitations for PPVI are RVOT dimensions over 22 mm with dynamic outflow aneurysms. Retrosternal conduits with extrinsic compression will have incomplete relief of stenosis [7]. In patients with conduits less than 16 mm a temporary relief of RVOT gradient may be achieved, however eventually these patients would need a larger effective diameter of the RVOT.
Between 09/2000 and 01/2007, 152 patients (pts) received a percutaneous pulmonary valve at Great Ormond Street Hospital for Children and The Heart hospital in London and at the Hospital Necker in Paris. Six out of 152 (3.9%) pts had to undergo ERS after a major complication during implantation of the percutaneous valve. Patient’s charts were analysed in retrospect. Their preoperative characteristics are summarised in Table 1 .
2.1 Case history 1
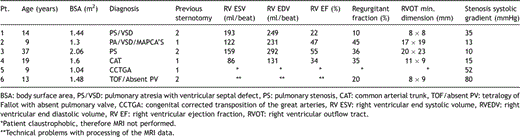
A 14-year-old male patient with a diagnosis of pulmonary stenosis/ventricular septal defect (PS/VSD) treated initially by balloon valvuloplasty and later a BT shunt in the neonatal period. He had complete repair at the age of 20 months with a 14 mm Hancock® valve and patch VSD closure. Later this was revised with a size 18 mm bicuspid homograft (from originally 25 mm). An 18 mm percutaneous valve was implanted. There was difficult catheter manoeuvring due to calcifications, but otherwise this was an uneventful implantation. Shortly after extubation, the patient became bradycardiac and hypotensive. Cardiopulmonary resuscitation (CPR) was commenced. A large left haemothorax was seen on fluoroscopy (Fig. 1 ). Two drains were inserted into the left thorax and auto-transfusion was performed. He was transferred to intensive care unit for further stabilisation, but it was decided to perform surgery because of persistent bleeding from chest drains. At operation, a tear was discovered at the left posterolateral aspect of the previously implanted homograft and the valved stent was removed. A size 22 Hancock® conduit was implanted and patch plasty of the proximal left pulmonary artery performed.
(a) Stent placed into calcified homograft. (b) Large left haemothorax with drains placed.
2.2 Case history 2
A 9-year-old male patient with a diagnosis of pulmonary atresia and VSD, major aortopulmonary collaterals (MAPCAs), anomalous origin of the left coronary artery (LCA) from right coronary artery (RCA). He had a left BT shunt and later complete repair using the base of the left atrial appendage to fill the distance between pulmonary artery bifurcation and the right ventricle. He also had a right-sided unifoculisation procedure. A 20 mm stented valve was implanted and was initially in a good position. Upon withdrawal of the delivery system, the stent became dislodged and was displaced into the RV. The patient remained haemodynamically stable during the entire procedure and was transferred directly into the operating theatre for stent removal and implantation of a homograft.
2.3 Case history 3
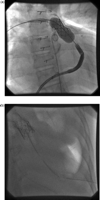
A 37-year-old female patient with a diagnosis of congenital pulmonary stenosis, had an open pulmonary valvotomy at the age of 5 years followed by homograft implantation at the age of 32 years. The indication for PPV was significant regurgitation of the previously implanted homograft. The procedure was uneventful and a size 22 mm valve was implanted but the patient became unwell approximately 4 h after the procedure with signs of low cardiac output. Chest X-ray demonstrated tilting of the valve (Fig. 2 ) with echocardiographic and clinical signs of severe right ventricular outflow tract obstruction. Adrenaline boluses were required to sustain cardiac output prior to transfer to the operating theatre. The right atrial pressure was measured at 40 mmHg shortly before cardiopulmonary bypass. At surgery, the percutaneous valve was replaced with a Hancock® conduit.
(a) Stent implanted in the RVOT. (b) Stent tilted obstructing the RVOT.
2.4 Case history 4
A 19-year-old female patient with a diagnosis of common arterial trunk (CAT) who had repair with a 12 mm pulmonary homograft in the neonatal period.
Before implantation, she had moderate stenosis and regurgitation with half systemic RV pressures. An 18 mm system was implanted, but an angiogram demonstrated no flow into the right pulmonary artery. Patient was transferred to the operating theatre with stable haemodynamics for explantation of the device and implantation of a pulmonary homograft.
2.5 Case history 5
A 9-year-old female patient with dextrocardia, congenitally corrected transposition (CCTGA), double outlet right ventricle (DORV), pulmonary stenosis, VSD. She had a Mustard procedure with VSD closure and homograft from the right ventricle to pulmonary artery at the age of 2 years. An 18 mm stent was later placed due to homograft stenosis at the age of 4.
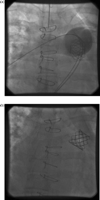
After deployment of an 18 mm percutaneous valve there was sudden LV failure. External cardiac massage was started. An aortic root injection demonstrated initially no flow into the LCA. After 25 min of cardiac massage, the stent became compressed and flow to the LCA was restored (Fig. 3 ). She was transferred directly into the operating theatre. The percutaneous valve was replaced with a size 22 mm pulmonary homograft.
(a) Stent implanted in homograft with occlusion of LCA on aortic contrast injection. (b) Flow into LCA restored after cardiac massage.
2.6 Case history 6
A 13-year-old female with the diagnosis of tetralogy of Fallot with absent pulmonary valve repaired in the neonatal period with closure of the VSD and implantation of a 16 mm pulmonary homograft. At repeated surgery at the age of 13 months, the homograft was replaced and her pulmonary arteries were plicated. She came for percutaneous valve implantation with significant homograft stenosis and moderate regurgitation.
A 20 mm device was chosen and deployed successfully. Shortly after implantation, her blood pressure started to drop. Repeat screening revealed a large right haemothorax. Drains were placed and in total 1900 ml blood was auto-transfused. She was transferred directly to the operating theatre for exploration. The valved stent was left in situ and the area of the junction between the right and main pulmonary artery reinforced with plicating sutures.
3 Results
3.1 Perioperative data of the six pts undergoing ERS are summarised in Table 2
Cardiopulmonary resuscitation requiring external cardiac massage and/or administration of adrenaline boluses to sustain cardiac output was necessary in three patients. Massive haemorrhage was treated with auto-transfusion in two patients; 1900 and 1000 ml was auto-transfused, respectively. Patients were transferred into the operating theatre after initial stabilisation in the catheterisation laboratory after a median of 30 min (30–320).
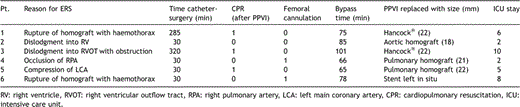
Rescue surgery: Cardiopulmonary bypass was instituted through femoral cannulation after surgical exposure of groin vessels in two out of six patients. All operations were performed under mild hypothermia (32–34 °C). The mean bypass time was 78 ± 13.4 min.
The percutaneous valve was explanted in five patients and was replaced with aortic or pulmonary homograft in three patients with standard technical principles of pulmonary valve replacement applied [1]. Two patients received a valved conduit (Hancock®, Medtronic Ltd.). In one patient, a contained defect in the homograft was presumed at the distal end of the homograft at the junction to the right pulmonary artery. This area was repaired with plicating sutures and the valve was left in situ.
Early outcome: All patients survived. One patient (patient 1) sustained a neurological insult consisting of bulbar weakness with speech difficulty and temporary upper limb weakness. On EEG, there were no signs of focal seizure activity and on MRI no gross abnormality could be revealed. The mean intensive care unit stay was 5.5 ± 3.2 days.
Follow-up: Follow-up was complete in all patients with a mean of 25.2 ± 17.1 months.
On echocardiography, all patients have competent pulmonary valves with a velocity of 2.3 ± 0.4 m/s (mean value). Patient 3 is on antiarrhythmic medication at follow-up after 3 years due to atrial fibrillation/flutter and is scheduled to have radio frequency ablation. One patient (patient 1) has mild neurological impairment with learning difficulties.
4 Discussion
Change in haemodynamic data following the implantation of a percutaneous pulmonary valve and also procedural and device related complications were described in previous publications [6,8]. Three significant early complications and seven minor complications were described in a group of 59 pts and this study also reported on device related adverse effects in 14 pts [8].
Mechanisms of acute PPVI failure: Upon reviewing the catheter implantation data, three different mechanisms of PPVI failure became apparent.
Firstly, there was dislodgement into a dilated right ventricular outflow tract in two of the patients. Another patient had a stent displaced into the distal pulmonary artery, which caused complete obstruction of the right pulmonary artery. With increasing experience in implanting percutaneous valve, patient selection and positioning of the device the incidence of this complication is likely to become less prevalent. Better understanding of complex geometry of the right ventricular outflow tract and its dynamics may also help to eliminate this complication mode [9].
Secondly, one patient suffered a compression of the LCA and as a result acute left heart failure. In retrospect, it became apparent that the previously implanted stent in the patient’s homograft had not been brought to its full dimension of 18 mm.
Currently, all patients undergoing implantation have an aortic root injection to delineate coronary anatomy and its relationship to the right outflow tract.
Thirdly, the last complication encountered in our cohort was a ruptured homograft with resulting massive haemorrhage. PPVI relies on a strong platform to anchor the stented valve, which is mostly provided by a calcified homograft placed at previous surgery. There is inevitable interfering with the integrity of the homograft in all stenosed conduits upon implantation, but this is contained by the stent or surrounding fibrous tissue. In our opinion, factors such as unusual angles in the outflow tract, use of bicuspidised homografts are prone to homograft rupture and haemorrhagic complication. With increasing experience and contrary to our previous belief, the presence of a heavily calcified homograft is not a risk factor for rupture.
Management of acute PPVI failure: Three of the patients described had a potentially life-threatening complication. Upon reviewing, the anaesthetic protocols and operation notes important patterns of management of the complications encountered emerged.
Blood products have to be cross-matched and readily available for emergent perusal. Auto-transfusion played an important role in management of two patients with haemorrhagic complications. It is imperative to establish a reasonable safety margin/platform before urgent transfer to the operating theatre.
Operations on failed percutaneous valve implantations require a highly skilled surgical team and can pose significant technical difficulties. Almost all patients would have had multiple previous operations. The access to groin vessel cannulation can be hindered by previous catheterisations or implantation device in situ. Furthermore, the choice of a pulmonary valve substitute with known limitations of homograft availability on an emergency basis can be problematic. Biological valves or heterografts are often the only option.
Multidisciplinary approach to decision making: So far surgical replacement of pulmonary valve represents the gold standard in majority of paediatric cardiac centres. Surgical pulmonary valve replacement in our institution is reserved for pts with dilated outflow tracts that may also require additional procedures [1]. The surgery for right ventricular outflow tract reconstruction and the morbidity associated with repeated sternotomies is well described [10]. Risk of cardiac laceration at repeated sternotomy has been reported to be around 5% [11]. Limitations of the surgical approach are the durability and availability of an ideal pulmonary valve substitute. However, the issue of durability of pulmonary valve substitute also applies to the percutaneous valve. It is a bovine jugular valve and there is no reason why it should not be susceptible to valve degeneration and calcification [7]. PPVI is now limited to conduit stenosis and/or regurgitation. Current research on right ventricular outflow tract reducer is likely to extend the indications of this procedure to higher proportion of patients who underwent previous repair of tetralogy of Fallot and conditions where a competent pulmonary valve is required [12,13].
Review of outcome in interventional procedures such as percutaneous atrial septal defect closure and percutaneous aortic valve placement demonstrates that the surgical community should be prepared and take an active part in hybrid and transcatheter procedures [14,15]. To provide the best care for each individual patient, we must free ourselves from preconceived ideas, accept facts, integrate novelty and built a team concept, whilst sharing experience and expertise across the artificial boundaries of disciplines [16].
5 Summary
Although some of the complications of percutaneous pulmonary valve implantation are related to a learning curve (4 among the first 50 pts and 2 among the last 102 pts) the need for emergency rescue surgery is unlikely to be completely abolished. Our experience highlights the importance of close collaboration between cardiologist and surgeons in these evolving technologies.
References
Author notes
Presented at the 21st Annual Meeting of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 16–19, 2007.
Disclosure: Dr Bonhoeffer is a consultant to Medtronic.
- cardiopulmonary bypass
- cardiologists
- pulmonary artery
- left coronary artery
- conduit implant
- brachial plexus neuritis
- catheterization
- pulmonary valve
- rupture
- surgical procedures, operative
- technology
- transplantation, homologous
- compression
- neurologic deficits
- right ventricular outflow tract
- percutaneous pulmonary valve implantation




