-
PDF
- Split View
-
Views
-
Cite
Cite
Moon Chul Kang, Chang Hyun Kang, Hyun Ju Lee, Jin Mo Goo, Young Tae Kim, Joo Hyun Kim, Accuracy of 16-channel multi-detector row chest computed tomography with thin sections in the detection of metastatic pulmonary nodules, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 3, March 2008, Pages 473–479, https://doi.org/10.1016/j.ejcts.2007.12.011
Close - Share Icon Share
Abstract
Objectives: The inaccuracy of conventional CT makes open thoracotomy and manual palpation inevitable in pulmonary metastasectomy. However, the introduction of multi-detector row CT technology made it possible to detect pulmonary nodules with a diameter of 1 mm. The purpose of this study was to investigate the accuracy of 1 mm thin-section 16-channel multi-detector row CT (TSMDCT) in the detection of metastatic pulmonary nodules. Methods: Twenty-seven patients who underwent pulmonary metastasectomy between November 2005 and September 2006 were included in the study. The primary tumors were colorectal cancer (n = 11), renal cell carcinoma (n = 5), osteosarcoma (n = 3), hepatocellular carcinoma (n = 3), thymic tumor (n = 2), bladder cancer (n = 1), thyroid cancer (n = 1), and primitive neuroectodermal tumor (n = 1). TSMDCT was performed in all patients in order to evaluate the location and number of metastatic nodules. The patients were divided into osteosarcoma and non-osteosarcoma groups, and the accuracy of TSMDCT was evaluated by comparison with the pathologic diagnosis of metastatic nodules. Results: A total of 117 nodules were detected preoperatively by TSMDCT scanning, and 198 nodules were resected during the operation. A total of 101 nodules were pathologically confirmed to be metastatic nodules. In the osteosarcoma group, the sensitivity, specificity, positive predictive value, and negative predictive value were 34%, 93%, 92%, and 38%, respectively. In the non-osteosarcoma group, the sensitivity, specificity, positive predictive value, and negative predictive value were 97%, 54%, 64%, and 96%, respectively. Subgroup analysis in the non-osteosarcoma group revealed that nodule size over 5 mm, number of metastatic nodules less than five, and disease-free interval over 24 months showed 100% sensitivity by preoperative TSMDCT. Conclusions: TSMDCT with 1 mm thickness image reconstruction showed high detection rate of metastatic pulmonary nodules in the patients with non-osteosarcoma. In highly selected subgroups, TSMDCT detected all the metastatic nodules which manual palpation could detect. Further study on the application of TSMDCT in thoracoscopic metastasectomy should be performed.
1 Introduction
Pulmonary metastasectomy has been an established treatment in selected patients with lung metastases from various solid tumors. Many studies have reported improved survival benefits of pulmonary metastasectomy in selected patients; however, no randomized studies have been reported to date [1–3]. Pulmonary metastasectomy is the generally accepted mode of treatment for patients with control of the primary tumor and with metastases confined to the lungs. Complete resection of metastatic nodules has been regarded as mandatory for long-term survival.
Minimally invasive surgery, such as video-assisted thoracoscopic surgery (VATS) metastasectomy, has been proposed for metastasectomy in highly selective patients [4–6]. Considering that the patients who undergo metastasectomy have a high possibility of systemic recurrence and additional adjuvant systemic therapies are anticipated, the VATS procedure is a valuable treatment option because the procedure can be performed with less morbidity and faster recovery. However, VATS metastasectomy has not been regarded as an adequate surgical procedure because manual palpation has been known to be inevitable in pulmonary metastasectomy. According to previous reports, more than 20% of additional nodules could be detected by manual palpation than by preoperative chest CT scan [7–9]. Due to the low sensitivity of conventional or helical CT, many consider VATS metastasectomy to be an incomplete surgery for the detection and removal of metastatic lung nodules. The inaccuracy of chest CT scans was primarily due to their inability to detect smaller nodules.
However, the recent development of multi-detector row CT technology has made it possible to detect smaller-sized pulmonary nodules with eminent resolution during a single breath hold and to reconstruct CT images at a thickness of 1 mm. In theory, this technology makes it possible to detect smaller nodules of up to 1 mm in size. We postulated that the acquisition of more detailed images using a chest CT scan could raise the sensitivity of the preoperative CT scan due to its ability to detect small-sized nodules that could be missed by thick-section CT scan. Therefore, we prospectively studied the results of 1 mm thin-sectioned multi-detector row CT (TSMDCT) in patients who underwent pulmonary metastasectomy. The aim of this study was to evaluate the accuracy of TSMDCT in the detection of metastatic nodules and to identify whether TSMDCT could be a substitute for manual palpation in pulmonary metastasectomy.
2 Materials and methods
A prospective study was performed in the 27 consecutive patients who underwent pulmonary metastasectomy with curative intent at our hospital from November 2005 to September 2006. This study was approved by the institutional review board of our hospital. The patients enrolled in the study (1) had the primary sites resected or controlled curatively, (2) were evaluated by TSMDCT within 1 month of pulmonary metastasectomy, (3) underwent pulmonary metastasectomy with curative intent, (4) were operated on by unilateral or bilateral thoracotomy, (5) without any evidence of infectious or other pulmonary disease, and (6) manual palpation was performed in all patients to detect metastatic nodules during operation. To compare the accuracy of TSMDCT according to cell type, the patients were divided into an osteosarcoma group and non-osteosarcoma group, and an analysis was performed according to the two patient groups.
All CT scans were acquired from the lung apices through the posterior costophrenic angle using a 16-detector row CT scanner (Sensation-16; Siemens Medical Systems, Erlangen, Germany). The scans were acquired using a detector configuration of 16 rows with 0.75 mm section thickness (16 mm × 0.75 mm), a beam pitch of 1.0, a gantry rotation time of 0.5 s, a tube potential of 120 kVp, and 120 effective mAs. The data were reconstructed into 1.0-mm-thick sections with 1.0-mm intervals using a high-resolution reconstruction kernel. Contrast enhancement was performed by intravenous injection of 90 ml of a contrast agent (Ultravist 370, Schering, Berlin, Germany) at a rate of 3 ml/s, followed by a 30 ml saline chase at a rate of 1.5 ml/s.
Two faculty radiologists who are specialists in thoracic imaging interpreted the CT scans in consensus. The transverse CT sections were read at a standard clinical CT viewing station (M-view; Infinitt, Seoul, Korea) in stacked cine mode at a window level of −700 HU and a window width of 1500 HU. The readers identified all pulmonary nodules in the osteosarcoma patients and all noncalcified nodules in the remaining patients on CT scans using a procedure similar to that used in routine clinical practice. The readers indicated the nodules with on-screen arrows and rated the confidence. The confidence grading was as follows: 5, definitely a metastatic nodule; 4, probably a metastatic nodule; 3, possibly a metastatic nodule; 2, unlikely to be a metastatic nodule; and 1, very unlikely to be a metastatic nodule.
All of the pulmonary metastasectomy procedures were performed by posterolateral thoracotomy. Bilateral metastasectomy was performed only in the cases in which bilateral metastasis was detected by TSMDCT. Exploration of the CT-negative side of the chest was not performed in this study. Sequential bilateral thoracotomy was performed at an interval of 2 weeks in the case of bilateral exploration. Additional chest CT scans were not acquired between operations. After isolated deflation of the involved lung, the whole lung was thoroughly palpated to detect metastatic nodules.
After careful palpation of the entire lung, the metastatic nodules were resected and numbered according to the numbering of the chest CT scan. Nodules that were detected by the surgeon but not by TSMDCT were marked separately. If the surgeon could not find the nodules that were depicted in TSMDCT, the nodules were recorded separately as missing nodules. All resected nodules were sent individually for histologic analysis. The recorded data included the number and location of nodules suspected preoperatively, the number and location of nodules palpated and resected intraoperatively, and the number, location, and size of the nodules that were confirmed to be malignant metastases on pathologic evaluation. The nodules detected by TSMDCT were compared with the nodules detected intraoperatively by analyzing the pathologic data in each case on a lesion-by-lesion basis.
Subset analysis was performed on a per-nodule basis. Since this strategy resulted in greater precision it served as an exploratory analysis to determine whether a unique subgroup could be identified. For all analyses, a p value of 0.05 was considered to be significant. Mann–Whitney test was used for the comparison of size between benign and metastatic nodules. A chi-square test was used for the histologic and maximum nodule size subset analyses. Ninety-five percent confidence intervals were calculated for all proportions. The sensitivity (the number of pathologically proven metastatic nodules detected on the preoperative TSMDCT divided by the total number of pathologically proven metastatic nodules), specificity (the number of pathologically proven non-metastatic nodules missed on the preoperative TSMDCT divided by the total number of pathologically proven non-metastatic nodules), positive predictive value (the number of pathologically proven metastatic nodules divided by the number of nodules detected on preoperative TSMDCT), and negative predictive value (the number of pathologically proven non-metastatic nodules divided by the number of resected nodules that were not detected on preoperative TSMDCT) were calculated in each group. In the non-osteosarcoma group, receiver operating characteristic (ROC) analysis was performed and an area under the curve (AUC) was calculated to evaluate the ability of TSMDCT to detect metastatic pulmonary nodules.
3 Results
The study group included 27 patients (9 females, 18 males; age range, 17–79 years; median age, 57 years). The primary tumors included were colorectal carcinoma in 11 patients, renal cell carcinoma in 5, osteosarcoma in 3, hepatobiliary carcinoma in 3, thymic tumor in 2, bladder cancer in 1, thyroid cancer in 1, and primitive neuroectodermal tumor in 1 patient. The disease-free interval (DFI) for the patients ranged from 0 to 213 months (median 22 months). DFIs less than 12 months were identified in nine patients, 12–24 months in eight patients, 24–36 months in six patients, and greater than 36 months in four patients. A total of 117 nodules were detected by preoperative TSMDCT. The number of metastatic nodules confirmed by radiologists (grade 4 or 5) was 94 (80%).
Unilateral thoracotomy was performed in 18 patients, and sequential bilateral thoracotomy was performed in 9 patients. Most of the metastatic nodules were resected by wedge resection or enucleation. Centrally located nodules were removed by lobectomy and segmentectomy in four and three patients. Six patients had received previous metastasectomy and underwent repeated metastasectomy for recurrence.
A total of 198 pulmonary lesions were palpated and resected during the operations. The average number of resected nodules was 5.8 (1–22 nodules) per thoracotomy and 7.3 per patients (1–39 nodules). Histological examination revealed 101 metastatic nodules, 96 benign nodules, and 1 bronchioloalveolar carcinoma. Of 96 benign nodules, there were 39 focal fibrosis, and 33 intrapulmonary lymph nodes, 1 chondroid hamartoma, and 23 completely normal lung parenchyma on pathologic examination. The size of the benign lesions varied from 1 mm to 15 mm (2.4 ± 2.4 mm). The size of the metastatic nodules varied from 1 mm to 45 mm (8.1 ± 8.8 mm). There was a significant difference in the size distribution of the metastatic and benign nodules (Fig. 1 ). The average size of the distribution of metastatic nodules in the osteosarcoma group was 3.7 mm. However, the mean sizes of the metastatic nodules other than the osteosarcoma were greater than 5 mm (Fig. 2 ).
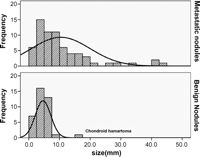
The size distribution graphs of benign and metastatic nodules. There was a difference in pattern of the size distribution between benign and metastatic nodules. The majority of nodules more than 10 mm diameter in size were metastatic nodules. The only one benign nodule that exceed 10 mm diameter in size was chondroid hamartoma.

Box plot for size distribution of metastatic nodules according to cell types. The size of the metastatic nodules in osteosarcoma was smaller than the sizes of other cell types. PNET: primitive neuroectodermal tumor.
Three of the patients in the osteosarcoma group underwent four thoracotomies, including one bilateral thoracotomy. A total of 46 nodules were resected during the operations, and 32 of the nodules were confirmed to be metastatic nodules by pathological review. However, only 12 of the nodules were detected by preoperative TSMDCT and 20 nodules were not detected by preoperative TSMDCT, but rather by manual palpation during surgical operation. Therefore, on a per-nodule basis, the sensitivity of TSMDCT was 34%, the specificity was 93%, the positive predictive value was 92%, and the negative predictive value was 38% in osteosarcoma group. On a per-patient basis, TSMDCT could not detect all of the metastatic nodules in any of the patients.
In the non-osteosarcoma group, 32 thoracotomies were performed in 24 patients, including 8 bilateral thoracotomies. A total of 152 nodules were resected, and 69 of those nodules were confirmed to be metastatic nodules by pathological review. Sixty-seven of the metastatic nodules were detected by preoperative TSMDCT and only two metastatic nodules were missed by preoperative TSMDCT. Therefore, on a per-nodule basis, the sensitivity of TSMDCT was 97%, the specificity was 54%, the positive predictive value was 64%, and the negative predictive value was 96%. The two missed nodules were detected during the operation in only one patient with renal cell carcinoma. Therefore, all of the metastatic nodules were detected preoperatively in 96% of the patients.
We evaluated the appropriateness of radiological dictation and the grading system by generating free-response receiver operating characteristic plots and calculating the sensitivity and specificity in non-osteosarcoma group. The ROC plots are shown in Fig. 3 . The area under curve was highest in the criteria of grade 4 (AUC 0.912, 95% confidence interval 0.855–0.951). These results suggest that the radiological grading system assigning a score grade 4 to metastatic nodules was appropriate in the non-osteosarcoma group. The sensitivity of the radiological diagnosis with the radiological grading system was 93%, and the specificity was 77%, the positive predictive value was 77%, and the negative predictive value was 93%. The values of sensitivity, specificity, positive predictive value, and negative predictive value of TSMDCT according to study group are given in Table 1 .
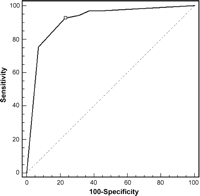
The ROC plots based on the radiological grading system for non-osteosarcoma group. The most accurate prediction was possible by the criteria of grade 4 (marked on ROC curve with a square symbol).
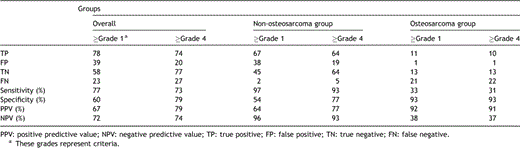
Diagnostic accuracy of TSMDCT in the detection of metastatic nodules
Subgroup analysis was performed for each metastatic nodule. The subgroups with nodule sizes over 5 mm, less than five metastatic nodules in preoperative TSMDCT, and DFI over 24 months showed 100% sensitivity in the non-osteosarcoma group, and all of the metastatic nodules were detected by preoperative TSMDCT if grade 1 was applied as the criteria for metastatic nodules. When grade 4 was used in the criteria, the sensitivity for the each subgroup was 98%, 97%, 100%, and 100%, respectively (Table 2 ).
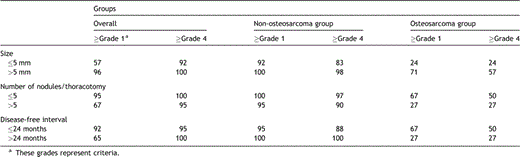
Subgroup analysis for the sensitivities of TSMDCT on a per-nodule basis in each patient group (data are expressed as sensitivity %)
4 Discussion
Pulmonary metastasectomy has been accepted as an established treatment option for the isolated pulmonary metastasis of extrathoracic malignancy. Although a randomized study has not been performed, many retrospective studies reported favorable long-term survival in the patients who underwent pulmonary metastasectomy [1–3]. One of the most important prognostic factors in pulmonary metastasectomy is complete resection. According to the report of the International Registry of Lung Metastasis, the 5-year survival of patients with complete versus incomplete resection was 36% and 13% in 5206 cases of pulmonary metastasectomy [1]. The importance of complete resection was also emphasized in other studies [3,10]. Therefore, in patients for whom pulmonary metastasectomy is planned, accurate preoperative detection of metastatic nodules is most important because underdetection of metastatic nodules could lead to an improper treatment plan. However, the reported accuracy of preoperative chest CT scan has not been satisfactory for the accurate detection of multiple metastatic nodules. Current evidences support manual palpation of metastatic nodules via thoracotomy or sternotomy as a standard procedure for the detection of metastatic nodules missed by preoperative CT scan.
However, there is another opinion regarding pulmonary metastasectomy. Due to the systemic nature of metastasis, the recurrence rate is more than 50% despite complete manual palpation. Therefore, some authors proposed that equal results might be achieved if only radiologically visible lesions were resected [11]. This hypothesis is based on the idea that the patients who will benefit from metastasectomy are those that have oligometastatic disease. They also point out that complete resection of all palpable metastatic nodules by open surgery is not a complete biologic resection of metastatic deposits [12]. This hypothesis was supported by several studies reporting comparable survival between VATS and open metastasectomy in selected patients [4–6].
However, the current standard and generally accepted procedure for pulmonary metastasectomy is open thoracotomy or sternotomy concomitant with careful manual palpation of the entire lung. Due to the low sensitivity of chest CT scan, the VATS procedure is not widely accepted at the present time. Many studies revealed that the detection rates of chest CT in pulmonary metastasis were not satisfactory. In a report from the International Registry of Lung Metastasis, the radiological assessment of the number of metastases was accurate only in 61% of patients, and it was underestimated in 25% of patients [1]. Later studies concerning the accuracy of helical CT for the detection of pulmonary metastatic nodules reported 69–82% sensitivity [7–9]. Based on the findings of these studies, open pulmonary metastasectomy is generally regarded as a more favorable and more accurate procedure than the VATS procedure.
However, the previous studies used thick-section chest CT image reconstruction. Most of those studies used 3–5 mm thickness image reconstruction. Because of motion artifacts and partial volume effects, CT may fail to show lesions with a diameter in the range of the slice thickness or smaller [13,14]. Paranjpe and Bergin [15] reported that the optimal visualization of structures in the lung parenchyma was obtained by slice thickness reduction to below 3 mm. However, recent advances in CT technology have made more thinly sectioned and detailed image reconstruction possible. As recently as 1998, the most advanced CT scanners were single-detector row CT scanners that required 25–30 s to image the entirety of the lungs with 7–10 mm slice thicknesses. Today, multi-detector row CT with 16-detector rows allows the entire adult lung to be scanned with 1-mm thick sections in as little as 5 s. This improvement has the potential to substantially increase the detection performance of metastatic pulmonary nodules, compared with that of single-detector row helical CT.
Several studies reported that the sensitivity of 1 mm thickness TSMDCT was higher than that of conventional helical CT. Fischbach and colleagues [16] reported that pulmonary nodules larger than 10 mm in size were equally detected with 5 mm and 1.25 mm thickness CT. Whereas lesions smaller than 5 mm in size were significantly better detected with 1.25 mm thickness CT. Wormanns and colleagues [17] reported that 5 mm thickness MDCT detected only 74–79% of the metastatic nodules detected by 1 mm thickness TSMDCT. However, these studies were merely comparisons between 5 mm and 1 mm thickness chest CT. Pathologic confirmation was not performed in those studies; therefore, the real sensitivity of thin-section chest CT for metastatic nodules was not evaluated in those studies.
Our study was conducted to evaluate the accuracy of TSMDCT by comparison with pathologic confirmation. The sensitivity of TSMDCT in our study was 97% on a per-nodule basis and 96% on a per-patient basis in the non-osteosarcoma group. Sixty-seven of the 69 resected metastatic nodules found by manual palpation were detected by preoperative TSMDCT. Only two additional nodules were detected in one patient. The sensitivity of TSMDCT in the non-osteosarcoma group was much higher than that of the pervious studies with thick-section chest CT.
However, the sensitivity of TSMDCT was disappointing in the osteosarcoma group. Only 12 of 32 metastatic nodules were detected by preoperative TSMDCT. The sensitivity of TSMDCT was 34% on a per-nodule basis. We assumed that the primary reason for the low sensitivity of TSMDCT might be that the nodules of metastatic osteosarcoma were much smaller in size and much greater in number than other histology. The low sensitivity of CT scan in osteosarcoma patients has already been reported in previous studies [18,19]. Although the number of cases in the osteosarcoma group was small in this study, the study results indicate that TSMDCT is not an accurate method in patients with osteosarcoma.
In the subgroup analysis of non-osteosarcoma patients, nodule size over 5 mm, number of nodules less than five, and disease-free interval over 24 months were associated with the complete detection of all metastatic nodules (100% sensitivity). The size and number of nodules are known to significantly affect the sensitivity of the CT scan. Margaritora and colleagues [8] reported that the overall sensitivity of helical CT for the detection of metastatic nodules was 82%; however, the sensitivity was 66% for lesions <10 mm but >6 mm and 48% for nodules smaller than 6 mm. Matsaerts and colleagues [4] reported that the detection rate of preoperative chest CT was high in patients with a single metastatic lesion and low in the patients with more than one lesion. We also assumed that a longer disease-free interval was associated with the oligometastatic feature of patients, which enhanced the sensitivity of TSMDCT. As a result, if indications of VATS metastasectomy are to be created by TSMDCT results, the aforementioned factors might be used as valuable parameters.
The radiologist’s diagnosis was evaluated by ROC curve analysis in the present study. In our study, grade 4 (probably metastatic nodule) was the most accurate cut-off value for the diagnosis of metastatic nodules, showing 93% sensitivity and 77% specificity in the non-osteosarcoma group. Although the radiologist’s diagnosis was more specific and accurate, the decrease in sensitivity would be inevitable if we were to follow it. Because the purpose of the study was to identify the overall sensitivity of TSMDCT itself, we used grade 1 for the data analysis, which represents all of the nodules detected by preoperative TSMDCT. Although it might seem evident that the sensitivity for the detection of metastatic pulmonary nodules would substantially increase, one drawback of the higher spatial resolution of MDCT is that many more transverse reconstructions are generated than with single detector CT. The interpreter must examine up to 10 times the number of images than previously had to be examined. As a result, the interpreter’s efficiency is adversely affected. Thus, current CT technology presents new challenges and opportunities that should fundamentally alter the paradigms with which lung scans are acquired and interpreted. We believe that computer-aided detection will play a key role in maximizing diagnostic performance when interpreting large, thin-section, multi-detector row CT scans. Computer-aided detection technology showed a substantially higher sensitivity rate than the conventional double reading [20].
Although the result of the thin-section MDCT was favorable in this study, the small number and heterogeneity of study population are limitations of this study. Especially, in osteosarcoma patients, the small number of study population could not draw any definite conclusion. Further studies including a larger study population will be necessary. In this study relatively large numbers of nodules were resected. Because the aim of this study was to verify the detection rate of TSMDCT, the authors tried to resect all palpable nodules during the operation. As a result many normal parenchyma or fibrotic lesions were resected, therefore, subsequent high number of resection compared with TSMDCT results and low specificity of manual palpation were inevitable.
In conclusion, TSMDCT with 1 mm thickness image reconstruction was a highly sensitive method and showed a very high detection rate of metastatic lung nodules compared with the historical reports performed by 3–5 mm-thickness chest CT. In the highly selected subgroups, TSMDCT did not miss any metastatic nodule in this study. It is our opinion that TSMDCT will be a promising assessment tool for the preoperative evaluation of pulmonary metastasis. Further study for the application of TSMDCT on thoracoscopic metastasectomy should be performed.
References
Author notes
Presented at the 15th European Conference on General Thoracic Surgery, Leuven, Belgium, June 3–6, 2007.
Financial support for this study was provided by the lung cancer research fund of Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine.
- osteosarcoma
- lung
- bladder cancer
- colorectal cancer
- carcinoma, hepatocellular
- renal cell carcinoma
- neuroectodermal tumors
- neuroectodermal tumors, primitive
- palpation
- preoperative care
- reconstructive surgical procedures
- thoracoscopy
- thoracotomy
- thymus neoplasms
- diagnosis
- neoplasms
- pulmonary nodule
- thyroid cancer
- chest ct
- metastasectomy
- diameter




