-
PDF
- Split View
-
Views
-
Cite
Cite
Adrian P. Businger, Harald Frick, Martin Sailer, Markus Furrer, A ciliated cyst in the posterior mediastinum compatible with a paravertebral Mullerian cyst, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 1, January 2008, Pages 133–136, https://doi.org/10.1016/j.ejcts.2007.09.036
Close - Share Icon Share
Abstract
Several types of cysts in the mediastinum have been described, such as bronchogenic or thymic cysts and esophageal duplications, most of which arise in the middle or posterior mediastinum close to the tracheobronchial system or the esophagus. We present herein the rare case of a ciliated cyst anatomically distant to the genitourinary organs. An abnormal formation in the posterior mediastinum was incidentally detected on chest X-ray during the preoperative evaluation for a herniorrhaphy in a 54-year-old woman who had a negative medical history. The patient exhibited no clinical signs or symptoms associated with the mass. MRI revealed hypointense signals in T1-weighted scans and homogeneous hyperintense signals in T2-weighted scans. The lesion was resected thoracoscopically and histologic and immunohistochemical stainings showed a ciliated cyst of probable Mullerian origin. Because of their uncertain biological behavior, cystic lesions in the posterior mediastinum should be removed surgically to allow definitive histologic diagnosis.
1 Introduction
Mediastinal cysts have a broad range of etiologies, such as bronchogenic, thymic, and neuroenteric cysts, or esophageal duplication [1]. Neuroenteric cysts may develop through abnormal separation of the embryonic germ cell layer [2]. In 2005, Hattori [3] described the first case of a ciliated cyst of probable Mullerian origin arising in the mediastinum, which is a very rare entity. Herein we report a patient with a ciliated cyst in the posterior mediastinum.
2 Case presentation
A 54-year-old, non-smoking woman under hormone replacement therapy with estrogen and gestagene (Trisequens) was found to have an abnormal shadow on chest X-ray (Fig. 1a and b ) obtained during a preoperative medical examination for an inguinal herniorrhaphy in 2006. The patient did not complain of respiratory problems or fevers. No anatomic abnormalities were detected. She had no history of weight loss, persistent cough, inhalation trauma, or overexposure to ambient or industrial agents. Physical findings and laboratory tests were normal. Chest MRI demonstrated a lesion, 28 mm × 40 mm × 45 mm in size, located in the posterior mediastinum, dorsal to the aorta. There was no direct communication between the lesion and the spinal cord, the tracheobronchial system, or the esophagus. The T2-weighted MRI scans showed a homogeneous hyperintense lesion (Fig. 1c); the T1-weighted scans showed a hypointense lesion (Fig. 1d). The left upper lobe was slightly displaced by the lesion. Of note, the lesion with the same dimensions existed on a chest X-ray that had been obtained in 1999. Surgical removal of the cyst was performed thoracoscopically, consistent with the radiologic diagnosis of a neuroenteric cyst. During the operation, the cyst was opened and an opaque, yellowish fluid was evacuated. The cyst had an extraordinarily thin wall with a smooth interior, and an exterior that was white and pale brown in color. The cyst was in close proximity to the vagus nerve.

(a and b) Chest X-ray shows an abnormal shadow. (c) T2-weighted scans reveal hyperintense signals with (*) slight suppression of the vertebral body. (d) T1-weighted scans showed a hypointense lesion in the posterior medistinum (coronal view).
Histologic examination of the specimen revealed a thin-walled cyst lined by regular cuboidal or cylindrical ciliated epithelium without cellular atypia (Fig. 2a–c ). Adjacent to the epithelium, there was an almost uninterrupted layer of smooth muscle, some slightly ectatic capillaries, fibrolipomatous stroma, and small peripheral nerves (Fig. 2c). The van Gieson stain (Fig. 2d) highlighted the smooth muscle layer, as did immunohistochemical staining with actin (Fig. 2e). The outside of the cyst was coated with a calretinin-positive mesothelium. Neither cartilage nor glands were detected.
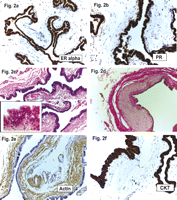
(a and b) The cyst epithelium showed strong nuclear positivity against estrogen receptor alpha, as well as against the progesterone receptor. The nuclei of the stromal cells, especially the smooth muscle cells, were completely negative for both ER and PR (ER alpha and PR; original magnification ×200). (c) The cyst almost had an extraordinarily thin wall with an almost completely circumferential smooth muscle layer (short arrows). With a higher magnification (insert HE; original magnification ×630) the ciliated epithelium is shown with regular round-to-oval nuclei without atypia and homogeneous cytoplasm of the cells. Focal formation of short pseudo-papillae is evident (long arrow) (H&E; original magnification × 200). (d) The smooth muscle layer is much more obvious and highlighted yellow with a van Gieson stain; as well as fibrous tissue stains red. Occasionally, the muscle layer is accentuated (arrow) with slight atrophy of the overlaying cuboidal epithelium (vG; original magnification ×200). (e) Immunohistochemistry underlines the circumferential smooth muscle layer; the walls of the small vessels (short arrow) stain positive as an internal positive control (Actin; original magnification ×200). (f) The luminal cyst epithelium was also strongly positive for cytokeratin 7 (CK7; original magnification ×400).
Additional immunohistochemical stainings were performed. As shown in Fig. 2f, the inner luminal epithelial layer showed a strong positive reaction against cytokeratin 7 (mouse K72; Cell Marque), the oncoprotein bcl-2 (mouse bcl-2/100/D5; Novocastra), and focal positivity against human epithelial antigen (mouse BerEp-4; Cell Marque). There was nearly 100% nuclear positivity for both estrogen receptor-alpha (ER; mouse 6F11; Novocastra) and progesterone receptor (PR; mouse PGR 312; Novocastra). None of the sections exhibited a positive reaction against cytokeratin 20 (mouse KS 20.8; Novocastra), the lung epithelial marker TTF1 (mouse 8G7 G3/1; Cell Marque), or the intestinal transcription factor CDX2 (mouse AMT 28; Novocastra). Based on Ki67 staining (mouse MM1; Novocastra), the proliferation rate of the cyst was estimated to be below 1%; p53 (mouse DO-7; Novocastra) showed weak nuclear positivity in about 30% of the epithelial cells. All incubations were performed on an automated Bond maX (Vision Biosystems, Novocastra) using ready-to-use pre-diluted antibodies. Together, these findings were compatible with a paravertebral cyst of probable Mullerian origin [4].
Postoperatively, the patient recovered well and was discharged from the hospital after 3 days and resumed her regular professional life after 2 weeks.
3 Comment
Mullerian cysts anatomically distant to the genitourinary organs or the pelvis are extremely rare disease entities [5–7]. Some authors contend that mediastinal paravertebral Mullerian cysts are more frequent than generally assumed and propose the establishment of mediastinal paravertebral cysts as an entity [4]. In 2005, Hattori [3] described the first case of a ciliated cyst of probable Mullerian origin arising in the mediastinum. The probable mechanism giving rise to Mullerian cysts in the mediastinum has not been elucidated. It is assumed that Mullerian cysts in the mediastinum arise during embryonic development vis-a-vis dispersed Mullerian epithelia or by metaplasia. Both radiologically and intraoperatively, the lesion in our case was diagnosed as a (neuro-) enterogenous cyst, or an esophagus duplication of a foregut cyst [8]. The expression of ER and PR and the histologic similarity to fallopian tubes with the ciliated cuboidal epithelium followed by a smooth-muscular layer revealed the Mullerian origin. Both enterogenous and bronchogenic cysts are lined by ciliated columnar epithelium with abutting smooth muscle. In contrast, the wall of bronchogenic cysts contains cartilage and glands and the wall of enterogenous cysts contains gastrointestinal tissue. It seems that mediastinal cysts are often misdiagnosed preoperatively as bronchogenic or neurogenic lesions, but when bronchogenic cysts are diagnosed based on the presence of cartilage and the hormone receptors are specified, this could be avoided. There are no systematic immunohistochemical studies pertaining to misdiagnosed Mullerian cysts.
4 Conclusion
The preoperative differentiation of lesions in the posterior mediastinum is challenging and asymptomatic cystic lesions in this location are often only detected incidentally. Since asymptomatic patients with mediastinal cysts may later develop malignant transformation and since the clinical course is highly unpredictable [1,9,10], we suggest resection of any lesion within the posterior mediastinum; a definitive histologic diagnosis can only be established following surgical removal.
Acknowledgment
The authors would like to acknowledge the effort put into this report by Thomas Boehm, MD and for providing the MRI pictures.




