-
PDF
- Split View
-
Views
-
Cite
Cite
Fabio Miraldi, Chiara Taffon, Michele Toscano, Antonio Barretta, Black cardiac paraganglioma in a multiple paraganglioma syndrome, European Journal of Cardio-Thoracic Surgery, Volume 32, Issue 6, December 2007, Pages 940–942, https://doi.org/10.1016/j.ejcts.2007.08.026
Close - Share Icon Share
Abstract
We describe a unique case of a patient with a ‘pigmented’ cardiac paraganglioma in a multiple paraganglioma syndrome. She was symptomatic for arrhythmias, hypertensive crises and dyspnoea due to a cardiac tumour, which was richly vascularised from the right coronary artery and was partially obstructing the right atrioventricular inflow. She was operated on, but the mass was not completely resectable due to its relationship with cardiac structures. The histological findings were paraganglioma with abundant, dark granular pigment. To our knowledge, pigment presence has never been cited in surgical cases of cardiac paragangliomas.
1 Introduction
Paraganglioma is an extra-adrenal location (10%) of pheochromocytoma and it is a neuroendocrine tumour which may arise from paraganglia of the autonomic nervous system located in the head, neck, retroperitoneum and thorax [1]. It is single in 90% of cases and multiple in 10%, resulting occasionally in 90% and familial in 10%. It is classically benign, slow growing and locally invasive. Cardiac paraganglioma is very rare, and considering that primary cardiac tumour incidence is very low (0.0005–0.5%), cases of this pathology are almost anecdotal.
Paragangliomas may be very rarely pigmented [2] and to our knowledge there is just one autoptic case of pigmented cardiac paraganglioma [3].
2 Materials and methods
A 69-year-old woman came to our department with previous history of surgical treatment for upper mediastinum paraganglioma (20 years before). She had a resection of a relapse in the neck 9 years later. Family history revealed that father and two brothers affected by neck paragangliomas were surgically treated. She was symptomatic for arrhythmias, hypertensive crises and dyspnoea, and consequently, transthoracic echocardiography showed an intracardiac mass located in the right atrium. Magnetic resonance imaging (MRI) showed an ovular paravalvular right atrial mass on the inferior wall with dimensions of 66 mm × 37 mm × 35 mm (Fig. 1 ). Coronary artery angiography showed that the mass was richly fed by a right coronary artery collateral (Fig. 1).
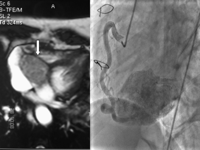
Cardiac MRI; white arrow indicates the paraganglioma, selected angiogram of right coronary artery.
On the basis of the symptoms and the previous surgical history we decided to operate on the patient. Operation was performed through a median sternotomy with cardiopulmonary bypass and moderate hypothermia with anterograde intermittent cold blood cardioplegia through the aortic root. The access was via right atriotomy from the base of right appendage to the inferior vena cava. Surgical findings were an intraparietal mass attached between endocardium and epicardium, partially obstructing the ostium of the coronary sinus and extending into the atrioventricular groove. We incised the atrial endocardium and on gross examination the lesion was soft, tan, with a smooth surface. We cut off a slice of the mass for extemporary anatomopathologic examination. The first report was compatible with melanoma metastasis, but further data of uncontrollable hypertensive crises during repeated cardioplegia delivery associated to the manipulation of the mass with patient’s history were highly suspicious for a new paraganglioma. The second extemporary report in fact was compatible with paraganglioma with dark, granular pigment. On the basis of this new report we attempted to resect the whole of the mass but were unable to resect more than a third of the tumour due to its relationship with cardiac structures. The weaning from extracorporeal circulation was complicated by hypertension controlled by α and β adrenergic blockade.
The postoperative course was complicated by right pleural effusion which was drained twice and the patient was eventually discharged on postoperative day 14.
3 Results
The definitive histological findings (Fig. 2 ) revealed the typical features of paraganglioma, with large polygonal cells arranged in nests and surrounded by sustentacular cells; the stroma was richly vascular. On immunohistochemistry the neoplastic cells were highly positive for neuroendocrine markers as neuron-specific enolase, chromogranin and synaptophysin. The sustentacular cells stained with S-100 antibodies.
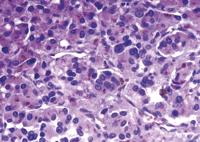
A photomicrograph of paraganglioma with large polygonal cells arranged in nests.
In addition, microscopic examination of the tumour showed the unusual finding of abundant, dark granular pigment within the neoplastic cells. The granules were positive for Fontana-Masson and negative for iron stains, consistent with melanin pigment. Immunohistochemistry with HMB45 antibodies gave negative results; no premelanosomes and melanosomes were identified by transmission electron microscopy; thus, the pigment was interpreted as neuromelanin.
The definitive histological result was compatible with pigmented cardiac paraganglioma (‘black paraganglioma’).
At 1-year follow-up on transoesophageal echocardiography and MRI the mass was still there (about 30 mm × 60 mm × 30 mm) with initial signs of intracardiac obstruction, but the patient was asymptomatic for dyspnoea and arrhythmias, normotensive on α and β blockers. Considering that these tumours are slow growing or sometimes bizarre and not growing at all we decided to just follow-up this case by MRI every 6 months. If the patient becomes symptomatic again we will consider a treatment by coronary feeding artery embolisation.
4 Discussion
Since 1974 [4] we know that cardiac pheochromocytomas involving the heart exist and that they can be excised, but they are very rare and even rarer is the concomitant presence of multiple locations. In our unique case we have a ‘black’ cardiac paraganglioma which was part of a multiple paraganglioma syndrome with familiar history of paragangliomas. Diagnosis was initially misled by the pigmentation of the mass (just one autoptic case of pigmented cardiac paraganglioma in literature). Intraoperative diagnosis was helped by manipulation of the mass and delivery of cardioplegia which caused tremendous hypertensive crises and that increased the doubt of neuroendocrine origin. Radical surgical resection is usually the treatment of choice [5], even with reconstructive surgery [6], but unfortunately our excision was not radical because the mass, despite not infiltrating, was locally very invasive. Transplantation was not indicated due to age (69 years old, HCV+), which is an alternative when total resection is not possible [7]. Consequently, we decided to be conservative on the basis of the concept that ‘a strategic retreat is better than a definitive defeat’.
The same wise conservative treatment was suggested by Turley et al. [8] who partially resected the mass, but was able to ligate the feeding arteries during surgery using colour Doppler on transoesophageal to check the interruption of the blood flow to the tumour. Ali et al. [9] preoperatively embolised an intrapericardial pheochromocytoma to facilitate the excision and to limit the catecholamine released during surgical manipulation, but the feeding vessels in this case were not coronary arteries. In fact, we do not plan to treat her with embolisation as long as the patient is asymptomatic in order to avoid a myocardial necrosis. However, we would consider such a risk if the mass were to grow again. Radiotherapy or chemotherapy is not, in fact, a good therapy if the mass has not been completely resected, even if it is described as a potential adjuvant cytotoxic chemotherapy on the basis of the same embryological origin of neuroblastoma and paraganglioma [10].




