-
PDF
- Split View
-
Views
-
Cite
Cite
Ottavio Rena, Luca Carsana, Silvia Cristina, Esther Papalia, Fabio Massera, Luca Errico, Cristina Bozzola, Caterina Casadio, Lymph node isolated tumor cells and micrometastases in pathological stage I non-small cell lung cancer: prognostic significance, European Journal of Cardio-Thoracic Surgery, Volume 32, Issue 6, December 2007, Pages 863–867, https://doi.org/10.1016/j.ejcts.2007.09.014
Close - Share Icon Share
Abstract
Objective: To determine the prevalence and prognostic significance of lymph node micrometastases and isolated tumor cells (ITC) in patients submitted for radical resection for pathological stage I non-small cell lung cancer (NSCLC). Methods: From January 1998 through December 2005, 87 consecutive pT1-2, pN0 NSCLC patients were enrolled. Surgical specimens were submitted to pathological routine examinations to define histotype, grade, stage, vascular invasion, necrosis and tumor proliferative index. A total of 694 regional lymph nodes were examined by means of serial sections stained with hematoxylin and eosin and labelled by immunohistochemistry (antibody AE1/AE3, DAKO). Relationships between these parameters and patients’ prognosis were investigated. Results: By histological examination, there were 36 squamous-cell carcinoma, 38 adenocarcinoma and 13 large-cell carcinoma. Micrometastases and ITC were detected in 19 lymph nodes (2.7%) of 14 patients (16%). Significant correlation was observed between micrometastases or ITC and adenocarcinoma (p = 0.03) and the absence of necrosis (p = 0.05). No relationship was demonstrated between micrometastases or ITC and T-status, vascular invasion or proliferative index (p > 0.05). Median follow-up was 3.2 (range 0.25–8.6) years. Two- and 5-year disease-free survival was similar for patients with and without micrometastases or ITC (79% and 64% vs 81% and 64%, respectively). Recurrence occurred in three patients with (two local, 66%) and in 21 patients without micrometastases or ITC (three local, 14%) (p = 0.186). By multivariate analysis only T-status was demonstrated to be a significant prognostic factor. Discussion: Micrometastases or ITC to regional lymph nodes are demonstrated to be not a rare aspect of pathological stage I resected lung cancer. In our series, the presence of lymph nodes micrometastases does not affect long-term disease-free survival.
1 Introduction
Lung cancer is a significant disease with approximately 170,000 new cases diagnosed annually in the United States. Of these, at diagnosis, 45% are limited to the thorax, where surgery is not only an important therapeutic modality but in many cases the most effective method of controlling the disease.
Even in patients with stage I non-small cell lung cancer (NSCLC), without an apparent systemic or regional lymph node metastases, a recurrent disease might develop within an early interval after the operation. Of all resected tumors, 28% are T1N0 and 29% are T2N0 lesions with a 5-year survival rate of 70% and 57%, respectively [1,2]. In our experience, in a series of 436 stage I resected NSCLC, we have observed a 5-year cancer-related survival rate of 63% and an incidence of recurrence of 40% perfectly according to the above-mentioned data [3].
Today in routine practice, pathological evaluation of resected NSCLC with particular focus on the detection of metastases in regional lymph nodes is done only by hematoxylin and eosin staining. Recent progress in immunohistochemical staining has detected keratin-immunoreactive tumor cells in up to 63% pathological negative intrathoracic lymph nodes from NSCLC patients [4–6].
The prognostic value of detecting these occult metastases in NSCLC and other tumors is the subject of intense research.
In 2002, the American Joint Committee on Cancer (AJCC) published the sixth edition of the AJCC Cancer Staging Manual including revised staging criteria for breast, colon and other tumors.
Staging for NSCLC remains unchanged [7]. The AJCC proposed an improved terminology for the classification of lymph node metastases introducing the concept of isolated tumor cells and micrometastases. Isolated tumor cells (ITC) are defined as single tumor cells or small clusters of cells, smaller than 0.2 mm in greatest diameter whereas micrometastases are clusters of tumor cells measuring between 0.2 and 2 mm in greatest diameter [7].
Currently, it is not clear whether the presence of ITC or micrometastases in the regional lymph nodes is a real prognostic factor in resected NSCLC and if it is associated with other clinical or pathological factors influencing prognosis.
The aim of this study is to determine the presence of occult metastases to the regional lymph nodes of patients undergoing surgical resection for stage I NSCLC; the presence of ITC or micrometastases, following the AJCC classification, is correlated to other factors such as stage, histology, mitotic rate, tumor necrosis, vascular invasion. Micrometastases and ITC to regional lymph nodes are indicated as factors influencing long-term prognosis as well.
2 Materials and methods
Of 343 consecutive patients with NSCLC who underwent radical surgery of the primary tumor with dissection of the hilar and mediastinal lymph nodes at the Thoracic Surgery Department of the University of Eastern Piedmont, Novara, Italy, during the period from January 1998 through December 2005, 87 patients had stage I disease (pT1–T2, pN0) identified by routine pathologic examination. All patients received cardiac and respiratory function tests before surgery. Clinical preoperative staging was performed by routine brain, chest and abdominal computed tomography scan (CT-scan), bronchoscopy and total body positron emission tomography fused with CT-scan (CT-PET): no one demonstrated extrapulmonary lesions suspected for either distant or hilar and mediastinal metastasis. Because of the CT-PET negative result, in particular at the level of the pulmonary hilum or mediastinum, routine mediastinoscopy was avoided. After pathological evaluation of the surgical specimens, CT-scan and CT-PET were evaluated and clinical preoperative stage I lung cancer was confirmed in each patient. From these 87 patients, we were able to obtain adequate paraffin-embedded lymph nodes and tumor specimens. The tumor stage was classified according to the Revisions in the International System for Staging Lung Cancer (1997) [8]. Paraffin-embedded sections of the pulmonary specimens were stained with hematoxylin–eosin (H&E) for histological classification and detection of tumor necrosis. When at microscopic examination, vascular embolization by tumor cells has been suspected, immunohistochemical staining with CD34 antibody was utilized to confirm the presence of vascular embolization. Each pulmonary specimen was then submitted to immunohistochemical staining with Mib-1 monoclonal antibody for the evaluation of the mitotic rate.
A total of 694 hilar and mediastinal lymph nodes from the 87 patients were sampled and analyzed. The H&E-stained histologic slides from formalin-fixed, paraffin-embedded hilar and mediastinal nodes have been reviewed. Based on the examination of the H&E histological slides, the initial nodal status of each patient was recorded. The lymph nodes were classified as pN0 according to current AJCC criteria for NSCLC [7]. Five serial sections, 4 μm in thickness, were made from each lymph node at 50 μm intervals; the first, third and fourth section stained with H&E were reviewed to confirm the absence of tumor cells and the second and fifth section were stained with immunohistochemistry for keratin AE1/AE3 (Chemicon Inc., Temecula, CA), using a standard DAKO Envision peroxidase method (DAKO, Carpinteria, CA). A working dilution of 1:2000 was used for the primary antibody. The detection of one or more keratin-positive tumor cells within the entire lymph node section, within the subcapsular or medullar sinuses was considered as certain evidence of tumor metastasis.
The sizes of detected metastases from the various lymph nodes from each patient were stratified into three categories: smaller than 0.2 mm (ITC), from 0.2 to 2 mm (micrometastasis), and larger than 2 mm (metastasis). The N status of each case was reclassified into six ordered categories, using the terminology proposed in the sixth edition of the AJCC cancer staging manual for breast cancer and other neoplasms: pN0, ITC, pN1mi, pN1, pN2mi and pN2 [7].
2.1 Follow-up and statistical analysis
After the primary surgery, the patients were examined every 3 months during the first 2 years, and every 6 months after the second year of follow-up. The evaluation included physical examination, chest, abdominal and brain CT-scan, blood chemistry and carcinoembryonic antigen assay.
All patients were followed up until death or December 15, 2006; no-one was lost at follow-up. The median observation period was 3.22 years (range 0.25–8.6). Disease-free survival was considered as the period between surgery and detection of tumor recurrence. Disease-related death was considered as death directly related to tumor relapse or to therapies administered for cancer cure, disease-related survival was considered as the period between surgery and disease-related death. Patients’ data were acquired from the inpatient and outpatient charts, by physicians and phone interviews. The association between the clinicopathological characteristics and the micrometastatic status was analyzed by a contingency table. Statistical significance was evaluated using the Chi-square test or the Fisher’s exact test. Survival curves were plotted according to the Kaplan–Meier method, and differences between the two curves were analyzed by the log-rank test. The Cox proportional hazards model was applied to the multivariate survival analysis. The difference was considered to be significant at p < 0.05. All data analyses were performed using Statistical Package for the Social Sciences (SPSS 11.0 for Windows; SPSS Inc., Chicago, IL).
3 Results
3.1 Clinicopathological characteristics and incidence of lymph node micrometastases
Among 87 patients affected by completely resected stage I primary NSCLC, there were 80 males and seven females with a mean age of 65.6 years (range 42–77). Histology included 36 squamous-cell carcinoma, 38 adenocarcinoma and 13 large-cell carcinoma of the lung. Twenty-six patients had stage IA (T1N0M0) and 61 had stage IB (T2N0M0) NSCLC. Visceral pleural infiltration was recorded in 20 out of 87 cases. Tumor necrosis was detected in 51 out of 87 specimens. Mean mitotic rate was 38.82% (range 5–80.37%). Vascular embolization detected by CD34 immunostaining was present in 20 cases out of 87 (22.9%).
Six hundred and ninety-four hilar or mediastinal lymph nodes were submitted to immunostaining with AE1/AE3 for the detection of micrometastases or ITC, with a mean of 7.97 lymph nodes/patients (range 4–15). Metastatic tumor cells were detected in 19 lymph nodes (2.7%) of 14 patients (16.3%); 10 cases had micrometastases or ITC within the hilar and four cases within the mediastinal lymph nodes. ITC were detected within hilar and mediastinal lymph nodes in seven and three patients, respectively, and within both hilar and mediastinal lymph nodes in one patient. Micrometastases were detected within hilar lymph nodes in three patients. Restaging of the patients nodal status, based on the anti-keratin immunostaining, was performed: among the patients with pN0 disease identified by conventional hematoxylin and eosin histopathologic study, 11 patients (12.8%) were restaged as ITC and three patients (3.5%) were restaged as pN1mi.
Patients affected by adenocarcinoma manifested an incidence of metastatic tumor cells detected at immunostaining (26.3%) significantly higher than those of patients affected by other tumor types (10/38 vs 4/49, p = 0.04); the presence of metastatic tumor cells showed to be significantly correlated with the presence of tumor necrosis (27%) (11/51 vs 3/36, p = 0.05). When the great dimension of the primary tumor is considered, 21 out of 87 tumors had a great diameter less than 2 cm and micrometastases or ITC occurred in four of these patients (19%), whereas tumors greater than 2 cm in diameter demonstrated an occult metastatic spread of 15.4% (10/66). In particular among adenocarcinoma patients, four cases out of 15 with a great diameter less than 2 cm manifested occult metastatic spread to regional lymph nodes (26.7%). Clinicopathological variables and their correlation with the presence of lymph nodes micrometastases or ITC cells are listed in Table 1 ; age, sex, T status, visceral pleural involvement, vascular embolization, mitotic rate are not correlated with the detection of micrometastases or ITC in the regional lymph nodes.
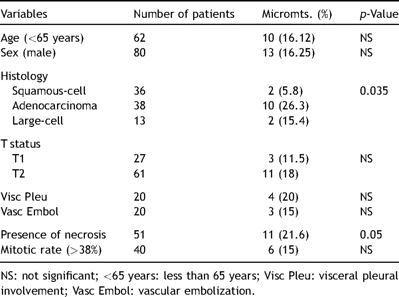
Clinicopathological characteristics of stage I lung cancer patients and association with lymph node micrometastatic tumor cells
3.2 Survival analysis, correlation with lymph node micrometastases, and factors influencing prognosis
The 5-year disease-free and disease-related survival rate for the entire cohort of 87 patients was 63.8% and 64.7%, respectively. We evaluated the influence of lymph node micrometastases or ITC detected by the cytokeratin immunohistochemical staining on patients’ disease-free and disease-related survival. During the follow-up period, three out of 14 ITC or pN1mi patients and 21 out of 73 pN0 patients experienced tumor recurrence. The median duration from surgery to recurrence in these 24 patients was 1.5 years (range 0.36–3.70). There was no difference between the time to recurrence in ITC or pN1mi and pN0 patients (2.04 years, range 0.53–2.46 and 1.42 years, range 0.36–3.70, respectively). The presence of micrometastases or ITC in regional lymph nodes was indicative but not significantly predictive of the pattern of recurrence: two out of three ITC or pN1mi patients (66.7%) manifested local recurrence whereas only three out of 21 pN0 patients (14.28%) had similar pattern of relapse (p = 0.184). The Kaplan–Meier survival curves showed that ITC or pN1mi patients and pN0 patients had similar disease-free and disease-related survival rates (Fig. 1 ).
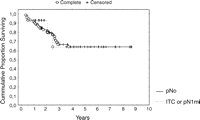
Disease-free survival analysis (Kaplan–Meier method) showing no significant differences in survivor function between patients with ITC and pN1mi from those with no nodal metastatic spread (pN0). Patients at risk were five and 38 in ITC or pN1mi and pN0 group at 2 years, respectively, and two and 15 in ITC or pN1mi and pN0 group at 5 years, respectively.
At univariate analysis, age, sex, visceral pleural involvement, vascular embolization, great tumor diameter, presence of necrosis, mitotic rate and presence of lymph node ITC or micrometastases did not affect long-term prognosis in the entire cohort. T status only resulted to be the variable significantly influencing long-term prognosis: 5-year disease-free survival was 76% and 56% for T1 and T2 (p = 0.048), respectively; 5-year disease-related survival was 80% and 57% for T1 and T2 (p = 0.021), respectively. Disease-free survival was influenced by histology (5-year survival rate was 63%, 59% and 44% for squamous cell, adenocarcinoma and large-cell, respectively).
At multivariate analysis, only T status resulted to be a factor significantly influencing long-term disease-free (p = 0.0022) and disease-related survival (p = 0.013); in particular the presence of lymph nodes micrometastatic tumor cells (ITC or pN1mi) was confirmed not to affect long-term survival.
4 Discussion
The most important prognostic factor to predict long-term outcome of patients affected by solid tumors, and therefore also by lung tumor, is if the tumor has spread locally and/or systemically. It has been demonstrated in the past that long-term survival (also for tumors in the pathological stage I and however small) is reasonably limited because of early relapse of the disease [2,3]. Occult local-regional or distant metastatic diffusion can also occur in patients submitted for complete removal of their primary tumor and this phenomenon is considered the basis of the late demonstration of overt metastases. The demonstration of the presence of distant metastatic foci is the rationale behind the use of adjuvant chemotherapy in these groups of patients with the purpose to improve long-term survival. Currently the pathological staging of cancer has mainly been based on the examination of the H&E-stained regional lymph nodes. Single tumor cells or small aggregates of tumor cells can also be overlooked among the lymphocytes and sinuses histiocytes and this could lead to a false-negative assessment. Different immunohistochemical techniques such as staining with Ber-Ep4, CAM-5.2, MNF116 or AE1/AE3 antibodies have been used to identify occult micrometastasis within the regional lymph nodes in breast and lung cancers [9–16].
In this paper we have demonstrated that in about 16% of patients submitted to complete surgical resection for stage I lung cancer, the lymphatic tumor dissemination is detectable by utilizing an immunohistochemical assay. Many papers in the previous literature have investigated this focus, reporting an incidence of occult lymph nodes metastatic spread between 4% and 27.8% [9,11–13,15–17]. These data are not easily comparable because series composition is highly variable by tumor stage, histology, and lymph nodal extension of dissection. By the same token, it is not easy to compare results of analyzed series published before 2002, when authors expressed their results about individuation of occult neoplastic spread by the generic expression of micrometastasis without specifying if it dealt with isolated tumor cells rather than with true micrometastasis according to the criteria of the AJCC [9–13,17]. Many authors in the past referred to the incidence of occult lymph nodal metastatatic spread and demonstrated its negative prognostic value, but in the majority of cases they did not analyze the variable stratifying their patients by pathological stage: many series analyze cohort of pT1–T4, pN0 patients but the prognostic value of micrometastases in stage I NSCLC alone is never analyzed [9–12].
The association of the presence of occult nodal spread has been correlated with many other variables: There is no accord within the international literature regarding factors increasing the probability of micrometastases in lung cancer: age, gender, T status, histology, proliferative index, grading of the primary tumor have been investigated with discordant results [9–17].
In our series, occult lymph nodal spread resulted more frequently in adenocarcinoma (despite the primary tumor diameter being greater or less than 2 cm) and associated with the presence of tumor necrosis. These data agree with those reported in the past literature that demonstrated a higher incidence of lymph nodal micrometastases in adenocarcinoma and large-cell carcinoma patients [9,11,15], even in small diameter tumors [13]. In 2002, Osaki et al. [15] demonstrated the incidence of lymph nodal micrometastases increasing from T1 to T2 primary NSCLC, without correlation with the histological subtype. Probably, all these differences are consequent to the small number of patients constituting the reported series.
The only element of agreement is that the more sophisticated the technical tool used, the greater the number of patients affected by tumor with lymph nodes occult metastases. So, the simultaneous utilization of more than one immunohistochemical assay technique can produce the detection of a higher number of patients with positive lymph nodes. In our study the use of a single antigen AE1/AE3 immunohistochemistry might have underestimated the real number of lymph nodal micrometastases.
Many papers have been published during the past decade, focusing on the impact of occult lymph node micrometastasis on patients’ survival. Some of these stated that the presence of micrometastasis significantly decreases the long-term survival and suggested the up-staging of patients with positive lymph nodes which could influence the indication for adjuvant therapies for a better disease control. Once more, which type of metastatic spread has the author observed: ITC or micrometastases? What is the impact of this variable on early stage NSCLC? To our knowledge only one paper clearly differentiates between isolated tumor cells and micrometastasis on the basis of the dimensional criteria suggested for breast cancer by the AJCC (authors analyzed 33 patients with pT1–2N0 disease) [16]. This last one and our paper agree with the fact that isolated tumor cells in regional lymph nodes did not affect long-term survival of stage I completely resected non-small cell lung cancer, and patients should not be up-staged on the basis of this characteristic.
Only two papers from the international literature specifically refer to lymph nodal micrometastases in pathological stage I NSCLC [13,15]. Wu et al. [13] referred to peripheral stage I adenocarcinoma and demonstrated a decreased long-term survival for patients with occult lymph nodal metastatic spread. Osaki et al. [15] identified a high incidence of occult lymph nodal metastatic disease in stage I NSCLC (27.8%), with an increasing rate from stage IA to IB (19% vs 38%), but detection of ITC or micrometastases in the stage IA (pT1N0) did not seem to affect long-term prognosis.
In our study, the presence of occult metastatic tumor cell in regional lymph nodes (ITC or pN1mi following the AJCC classification) was not demonstrated to be significantly associated with a survival difference compared with cases that remained node negative after immunohistochemistry. Patients at pathological stage I NSCLC with and without occult isolated tumor cells within the regional lymph nodes have the same poor long-term prognostic rate: so the identification of ITCs within the regional lymph nodes cannot explain the limited prognosis for early stage lung cancer.
During the past decade, some authors investigated the possibility of coexistence of lymph nodes and bone marrow occult micrometastasis by using immunohistochemical staining. Surprisingly, they observed that patients with lymph nodal occult metastasis may or may not have associated bone marrow occult metastasis and the presence of one of these conditions did not affect the other one or the long-term survival. Lymph node metastatic spread seemed to increase the possibility of a hematogenous recurrent disease, whereas bone marrow metastatic spread did not affect survival [12,15]. So, probably, the mechanisms ruling lymph nodal and hematogenous cancer spread are different and differently influence the disease progression. However, the utility and significance of detecting occult tumor cell dissemination remain controversial, laborious and time-consuming. Its prognostic value should be confined to large-scale multi-institutional clinical trials because it might have an important effect on the future management of lung cancer.
References
Author notes
Presented at the 15th European Conference on General Thoracic Surgery, Leuven, Belgium, June 3–6, 2007.
The study was performed within the Research Fellowship Program ‘Dottorato di Ricerca in Tecnologie e Terapie Avanzate in Chirurgia’, appointed by ‘Tor Vergata’ University, Roma, Italy. Authors wish to thank ‘Fondazione FERRERO ONLUS’ for its support to research against cancer in Italy.




