-
PDF
- Split View
-
Views
-
Cite
Cite
Parwis B. Rahmanian, Farzan Filsoufi, Javier G. Castillo, David H. Adams, Video-assisted trans-mitral left ventricular false aneurysm repair with a septal occluder, European Journal of Cardio-Thoracic Surgery, Volume 32, Issue 5, November 2007, Page 799, https://doi.org/10.1016/j.ejcts.2007.07.030
Close - Share Icon Share
A 41-year-old male with a history of myocardial stab-wound, which was repaired with a patch through a sternotomy 25 years ago, presented with a large calcified false aneurysm at the left ventricular antero-lateral free wall (Fig. 1 ). He underwent a video-assisted trans-mitral false aneurysm repair using a septal occluder (Fig. 2 ).
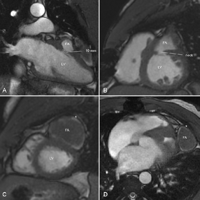
Cardiac magnetic resonance imaging (MRI) showing a large false aneurysm communicating with the left ventricle through a small neck which measures 10 mm × 7 mm. The left ventricular antero-lateral free wall is completely covered by a calcified shell (10 cm × 4 cm) forming the external layer of the false aneurysm. Left and right ventricular size and function were normal. Opening the cavity of the false aneurysm to get access to the neck was deemed prohibitively hazardous. Therefore a trans-mitral approach was elected. (A: two-chamber view, B and C: apical view; D: four-chamber view; FA: false aneurysm, LV: left ventricle, *: calcified shell).
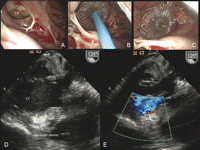
A left atriotomy was performed. Following the retraction of the anterior mitral leaflet and using a 60° angle scope, the neck of the false aneurysm was identified (A). Direct surgical repair was attempted without success because of (1) difficulty to reach the surgical site with long surgical instruments and (2) fragility of ventricular tissue at the inferior edge of the defect. These major technical challenges led us to apply a percutaneous occluder-device under video-assisted vision. A 12 mm Amplatzer septal occluder-device was placed under video-assisted vision across the neck of the false aneurysm with the left disk in the pseudoaneurysm and the right disk on the ventricular side (B and C). Transesophageal echocardiography showed a definite closure of the false aneurysm (D and E). The patient was discharged on postoperative day 7 and is doing well 2 months following the procedure. Surgical repair of FA using a patch is the appropriate treatment to prevent peripheral embolization, ventricular arrhythmia, and late rupture. Occasionally the surgical approach to the site of intervention can be complicated due to extensive calcification. In this scenario, a trans-mitral approach with the use of an occluder-device may be a valuable therapeutic alternative. In this case this procedure was performed easily under vision in a very short time. Currently occluder-devices are used with success for atrial septal defect repair and are not approved for such indications. Further studies with larger number of patients are required to determine their role in the treatment of FA as a ‘rescue therapy’ when conventional surgical treatment is unsuccessful. (A–C: intraoperative views; A: trans-mitral visualization of the false aneurysm (FA) using a 60° angle scope; B: delivery of Amplatzer occluder device, C: occluder device in place; D–E: intraoperative echocardiography; D: modified transgastric view; E: color Doppler; LV: left ventricle).




