-
PDF
- Split View
-
Views
-
Cite
Cite
Sanjay Asopa, Anish Patel, Omar A. Khan, Rajan Sharma, Sunil K. Ohri, Non-bacterial thrombotic endocarditis, European Journal of Cardio-Thoracic Surgery, Volume 32, Issue 5, November 2007, Pages 696–701, https://doi.org/10.1016/j.ejcts.2007.07.029
Close - Share Icon Share
Summary
Non-bacterial thrombotic endocarditis (NBTE) is a disease characterised by the presence of vegetations on cardiac valves, which consist of fibrin and platelet aggregates and devoid of inflammation or bacteria. NBTE has increasingly been recognised as a condition associated with numerous diseases and a potentially life-threatening source of thromboembolism. NBTE is not a common entity; however it is frequently underestimated, probably due to underlying diseases (cancer, autoimmune disorders, HIV). NBTE is difficult to diagnose and relies on strong clinical suspicion. NBTE is also difficult to manage and each case should be individually managed by identifying and treating the underlying pathology. Surgical intervention is not recommended unless the patient is in acute congestive failure.
1 Introduction
Non-bacterial thrombotic endocarditis (NBTE) is a disease characterised by the presence of vegetations on cardiac valves, which consist of fibrin and platelet aggregates and are devoid of inflammation or bacteria. NBTE was first described, in 1888, by Zeigler [1], who introduced the word thromboendocarditis to describe deposition of fibrin on cardiac valves. It was Gross and Friedberg [2] in 1936, who coined the term ‘nonbacterial thrombotic endocarditis’.
Over the years NBTE has increasingly been recognised as a condition associated with numerous diseases and is a potentially life-threatening source of thromboembolism to the brain, heart, spleen, kidney and other important organs. In this review we discuss the epidemiology, pathophysiology, clinical features and the evolving role of investigations which now make it possible to establish an ante-mortem diagnosis.
2 Epidemiology
In approximately 1.2% of all autopsy patients, vegetations caused by NBTE may be found, although the reported incidence of NBTE ranges from 0.3% to 9.3% [1]. Kuramoto et al. [4] found the highest prevalence (9.3%) and suggested that previous studies underestimated the prevalence due to inadequate pathological evaluation of specimens; NBTE vegetations vary in size, and are quite friable and easily embolised. NBTE most commonly affects patients from the fourth through the eighth decade of life [3–5], though all age groups can be affected [1]. The sex distribution of NBTE is equal among males and females [1,3–4,6]. The incidence of visceral embolism varies from 14.1% to 90.9% [1,5]. This may be partly due to the diverse criteria used by authors to define embolic events clinically and pathologically.
NBTE has been documented in patients with advanced stage malignancy, either solid [7] or haematological [8]. In a prospective echocardiography study [7] of 200 non-selected patients with solid tumours, NBTE was noted in 19% compared to 2% in the control group. It may also complicate other chronic diseases such as tuberculosis, uraemia [9], and acquired immune deficiency syndrome [10]. Other disorders NBTE is associated with include connective tissue disorders, autoimmune disorders and hypercoagulable states [11]. A number of recent echocardiographic studies with large numbers of patients have confirmed that systemic lupus erythematosus (SLE) patients possessing antiphospholipid antibodies (aPL) have a higher prevalence of vegetations, particularly on the mitral valve, than those without [11,12]. Trauma from indwelling pulmonary catheter, central venous catheter [1], snake bite [13], overdose [14] and late effect of radiation therapy have also been associated with NBTE.
3 Pathogenesis
Although the aetiology and pathogenesis of NBTE is not fully elucidated, several mechanisms play a role. The common factor is endothelial damage and subsequent exposure of the subendothelial connective tissue to the circulating platelets. The pathogenesis can be divided into factors initiating NBTE and the subsequent development of verrucae. Factors implicated in the initiation are: (a) immune complexes, (b) hypoxia, (c) hypercoagulability and (d) carcinomatosis.
3.1 Immune complexes
Williams [15] in 1980 implicated circulating immune complexes in the formation of NBTE. Since then immunoglobulin and complement deposition have been demonstrated at the base of NBTE vegetations through immuno-histochemical techniques [11].
Libman-Sacks endocarditis is a form of NBTE seen in patients with connective tissue disorder and involves the formation of fibrin-platelet thrombi on the altered valve, re-organisation of which leads to valve fibrosis, and dysfunction [16–17]. Libman-Sacks valvular lesions, as described in early pathological studies, are sterile fibrofibrinous vegetations that may develop anywhere on the endocardial surface of the heart but with a propensity for the left valves, particularly the ventricular surface of the mitral valve. They are typically sessile, wart-like, and small; varying from pinhead size to 3–4 mm [11,12]. The valvular lesions in patients with aPL occur as valve masses (nonbacterial vegetations) or thickening. These two morphological alterations can be combined and are thought to reflect the same pathological process. It has been postulated that aPL mediates valvular damage merely by promoting thrombus formation on the injured valve endothelium [11].
3.2 Hypoxia
Studies have linked hypoxia with NBTE. Nakanishi et al. [18], in a rodent model, studied the affect of hypoxia on the development of NBTE. Rats were subjected to a hypoxic environment simulating high-altitude dwelling (equivalent of 5500 m elevation). Tissue factor (TF) activity and morphological changes in the mitral valve were measured using immunohistochemical and immunoelectrical methods. The incidence of NBTE at 4, 6, 8, 12 weeks of exposure to hypoxia was found to be 33%, 29%, 65% and 100%, respectively. Significantly higher levels of plasma TF activity were noted compared to the controls. There was significant correlation between plasma TF activity and the incidence of NBTE. Immunoreactivity for TF protein was detected in macrophages, stromal cells, in a few endocardial cells, extracellular matrix of the cardiac valves, and also in the macrophages within the thrombus.
Truskinovsky et al. [19] reviewed 50 autopsied patients with NBTE and compared them with 50 age, race and gender-matched control patients without NBTE. They found that patients with NBTE had heavier lungs; clinically and histologically more severe pulmonary disease. There was no statistical difference in the extent of metastatic spread in the NBTE group compared to the control. They concluded that in some patients, hypoxia could lead to NBTE, possibly through altered coagulation states.
Similarly Dutta et al. [20], in their retrospective study on a cohort of cancer patient with cerebral embolism, detected NBTE in 18% of patient population and found that smokers had a higher incidence compared to non-smokers. Persistent hypoxic insult may be implicated in activation of TF, which leads to a hypercoagulable state, predisposing to NBTE in this patient population. Whether mechanisms other than hypoxia, that activates TF or increases the activity of TF, have an important role to play is yet to be elucidated.
3.3 Hypercoagulability
MacDonald and Robbins [3] in 1956 postulated that valvular degeneration and hypercoagulable state were important in the genesis of NBTE. Trousseau first noted the association between thrombosis and malignancy. Associated clotting abnormalities include hypofibrinogenemia, hyperfibrinogenemia, thrombocytopenia, decreased concentration of factors V, VIII and XIII, circulating fibrin degradation products, decreased anti thrombin III [1,21], increased turnover of platelets and accelerated fibrinolysis [1,21]. Kim et al. [22] found histological evidence of disseminated intravascular coagulopathy (DIC) in 50% patients with NBTE.
3.4 Carcinomatosis
The association of carcinomatosis and NBTE is well established. Mucin-producing adenocarcinoma from the gut and lung and disseminated intravascular coagulation (DIC) are commonly associated with NBTE [1,23]. Bedikian et al. [23] showed in a study of 31 cases with malignant neoplasm and NBTE that 71% of patients with NBTE had concomitant DIC. Adenocarcinomas of the lung or ovary were the most common tumours (48%), followed by haematologic malignancies (25%).
However, in a recent series from Mayo clinic [24], 30 patients with NBTE were identified from the valvular specimen of patients who underwent valve surgery. Immune mediated disorders were identified in 18 (60%) patients. No patient had malignancy or DIC. In contrast to the previous autopsy series, interestingly NBTE in the surgical population was more commonly associated with autoimmune disorders than malignancy and/or DIC.
Although the relationship between NBTE and DIC is still unclear, DIC is characterised by concomitant systemic activation of thrombosis and fibrinolysis to which abnormal TF activity is pivotal. TF may be secreted into circulation by promyelocytic leukaemia cells [25], expressed on the surface of adenocarcinoma cells, and cytokines (TNF and interleukin 1) which enhances the expression of TF by endothelial cells [26], thus leading to a prothrombotic state. This in turn may lead to damage of a previously normal valve and possible vegetation formation [19].
4 Clinical features
There are no pathognomonic signs and symptoms that allow for the diagnosis of NBTE. Previously Mckay and Wahler [27] outlined a triad consisting of: (1) a disease process known to be associated with NBTE, (2) presence of a heart murmur and (3) evidence of multiple systemic emboli. However, vital to the diagnosis is the high clinical suspicion of NBTE in a patient being treated for infective endocarditis (IE) and not clinically progressing.
A cardiac murmur is a poor sign for NBTE. The presence of a new murmur or a change in a pre-existing murmur is more helpful, but occurs infrequently. Nevertheless, a murmur noted in the setting of malignancy, DIC or patients with known antiphospholipid syndrome should alert the clinician to consider NBTE.
It is essential to differentiate NBTE from infective endocarditis (IE) (Table 1 ). In patients with IE, the classic textbook signs along with peripheral stigmata of IE (Osler’s nodes, Janeway lesions) are increasingly uncommon in the western world. However, vasculitic phenomena such as splinter haemorrhages, Roth spots, and glomerulonephritis are common. Thromboembolism to brain, lung, or spleen occurs in up to 30% of patients and is often the presenting feature [28]. These features may be present in NBTE due to systemic embolisation rather than the vasculitic phenomena encountered in IE.
Recurrent emboli should be considered as a hallmark feature of NBTE, occurring in up to 50% of patients [1], with a tendency to embolise to the brain, kidney, spleen, mesenteric bed or the extremities. Fresh vegetations readily dislodge and embolise. The clinical presentation can be from valvular insufficiency due to destruction of the valve leaflets or stenosis due to the size of the vegetation [29] but is uncommon.
5 Diagnosis
The diagnosis of NBTE is considerably more elusive than that of IE. There are no markers of the bloodstream and the vegetations are small, easily friable and frequently embolise, leaving only small remnants to be identified on the valve. Cardiac murmurs, a hallmark of IE, are frequently absent, and there is some evidence that echocardiography is less sensitive for the detection of NBTE than it is for IE [1]. Nevertheless, with recent advances in technology, ante-mortem diagnosis of NBTE is possible.
5.1 Echocardiography
Advances in echocardiographic techniques such as harmonic imaging, on-line digital storage has allowed ante mortem diagnosis and anticipation of potential complications. NBTE vegetations are typically small, <1 cm in diameter, broad based and are irregular in shape [9]. Transoesophageal echo (TEE) has a higher sensitivity (90%) for vegetation diagnosis to transthoracic echo (TTE), especially for vegetation of <5 mm. Given that NBTE vegetations are often small and irregular; a TEE should be ordered if the clinical index of suspicion for NTBE is high.
In a prospective TTE study of 200 cancer patients by Edoute et al. [7], cardiac valvular vegetation were found in 19% (38) of the study group compared with only 2% (2) in the control arm. Left-sided heart valves were more commonly involved (mitral more than aortic). Thromboembolism was found in 11% of the study group. Of the 19% (38) with valvular vegetations, embolic phenomenon was found in 24% (9) compared to 8% (13) in 162 patients. Two-dimensional (2D) and M-mode Doppler was used for this study. Potentially, the incidence of NBTE may have been higher if TEE was utilised.
Dutta et al. [20] in a retrospective study examined the frequency of cardioembolic findings in consecutive patients with cancer referred for TEE evaluation of cerebrovascular events. Although the study cohort comprised of 51 patients, of whom 18% had NBTE vegetations, and 47% and 55% of who had definite, and definite or probable cardiac sources of embolism, they documented for the first time a high frequency of NBTE and other cardio-embolic sources in patients with cancer and cerebrovascular events selected for TEE.
TEE has been used in predicting the embolic event in IE. Salvo et al. [30] suggested that right-sided endocarditis, staphylococcal infection, vegetation length (>15 mm), and mobility were predictors of embolic events. However, this cannot be assumed for NBTE, since the NBTE vegetations are smaller, more friable and asymptomatic embolisation is frequent.
The use of TEE as a screening tool in the prevention of cerebrovascular events in susceptible patient populations is yet to be elucidated. As yet no clear morphological features of NBTE vegetation have been described that predicts embolic risk. Moreover, one cannot confidently distinguish between NBTE and infective vegetations from an echocardiogram. The diagnosis must always be based on the clinical context.
5.2 Laboratory tests
Comprehensive haematological and coagulation studies; DIC should be looked for with full blood count, prothrombin time, partial thromboplastin time, fibrinogen, thrombin time, D-dimers and cross-linked fibrin degradation products. These may be normal, but in the setting of carcinoma an abnormal result should alert the physician to a diagnosis of NBTE. Multiple blood cultures should be undertaken to rule out any infective cause, although negative blood cultures can be frustrating and can occur in 2.5–31% of all cases of IE [31]. Negative blood cultures may be a result of antibiotic therapy but also due to infections by fastidious organisms requiring special tools including serology for bacterial and fungal causes. They include, among others, coxiella, legionella, chlamydia, HACEK group, and fungi (candida, histoplasma, and aspergillus species).
Immunological assays for antiphospholipid syndrome (APS) should be undertaken in patients presenting with cerebrovascular events and recurrent systemic emboli. aPL is detectable by two kinds of tests: (1) solid phase immunoassays, typically ELISA, with phospholipids used as the coating antigens, or (2) phospholipid-dependent coagulation tests, by virtue of the ability of some aPLs to impair in vitro coagulation reactions, thereby prolonging clotting time [11,12]. Some authors have suggested that patients with primary APS with anti-cardiolipin antibody (aCL) titre >40 GPL have a higher risk for thromboembolic events [12].
Polymerase chain reaction (PCR) using nucleic acid target or signal amplification along with sequence analysis allows for a rapid and reliable detection of culture negative endocarditis from blood and tissues of patients with infective endocarditis allowing differentiation from NBTE. PCR has been proposed as a major Duke diagnostic criterion [31–33]. Its value also lies in the fact that PCR can be used as a tool when phenotypic characterisation is necessary subsequent to the isolation of multiple organisms in separate cultures (i.e. by contamination with skin flora during sampling or polymicrobial infection in intravenous drug abusers). Specific primers are now available for many bacterial agents including Tropheryma whippelii, Coxiella burnetti, and species of Bartonella, Chlamydia, Brucella, Legionella, Mycobacteria and Mycoplasma. Future improvements include quantitation by real time PCR for faster, more accurate results, and the possibility of investigating common antimicrobial resistance genes enabling a targeted approach to antibiotic treatment [31,34].
5.3 Cardiac magnetic resonance imaging
There is a paucity of literature regarding the diagnosis of endocarditis by cardiovascular MRI, which is increasingly undertaken to assess cardiac function, valve function and morphology. Two case reports have been published using this modality for the diagnosis of valvular vegetations on the aortic and the mitral valves. In the case of the aortic vegetation [35], fast GE (Fastcard) delineated the vegetations as areas of low signal at the valve leaflets in contrast to the bright, flowing blood. In the case of the mitral valve [36], fast gradient echo sequence with steady-state free precession (TrueFISP) was used which demonstrated these vegetations on the posterior and the anterior valve leaflets. The excellent resolution of cardiovascular MRI using TrueFISP gradient-echo sequence allows the morphology of small heart structures such as valves to be examined in detail.
This may make more invasive procedures such as TEE unnecessary for the diagnosis and follow up of endocarditis. MRI may therefore be useful in cases with apparently normal valves on TEE but in whom there is a high clinical index of suspicion. This strategy may also be adopted in suspected cases of NBTE although to date, no reports using the cardiovascular MRI have been published.
Diffusion-weighted MRI (DWI) [37], a relatively new imaging technique, may differentiate cardioembolic stroke caused by infective endocarditis or by NBTE. Using DWI, it is possible to detect ischaemic events within a few minutes of onset of symptoms. Due to its high signal-to-noise ratio it can detect very small ischaemic lesions. In a small study of 35 patients (27 episodes of IE, 9 episodes of NBTE), definite differences in the stroke patterns were exhibited by NBTE compared to IE. The DWI lesions were classified into four patterns. NBTE exhibited pattern 4 (multiple small and medium or large disseminated lesions). IE lesions exhibited pattern 1 (single lesion), 2 (territorial infarction), 3 (multiple punctuate disseminated) and also pattern 4. Due to the pattern heterogeneity between IE and NBTE stroke, DWI imaging may be used to differentiate and diagnose NBTE from IE. These differences may be due to the lack of cellular component in NBTE emboli with their tendency to undergo fragmentation and cause multiple lesions (Fig. 1 ).
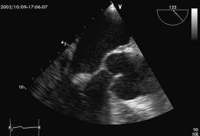
Transoesophageal echocardiogram, 120 degree view at mid oesophageal level. There is a small mobile mass seen at the tip of the anterior mitral valve leaflet. This oscillates independently to the valve, the hallmark of vegetation.
5.4 Histology
The vegetations of NBTE are friable, white or tan masses, usually situated along the lines of the valve closure, on the leaflets, which may be normal or previously damaged. They vary greatly in size from tiny lesions to large and exuberant masses. NBTE consists of degenerating platelets interwoven with strands of fibrin and forming a bland, featureless eosinophilic mass except for a few trapped leucocytes (Figs. 2–4 ).
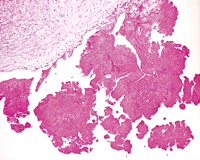
Histological section through the part of mitral leaflet. The vegetations appear to have a uniform eosinophilic appearance. There are no significant inflammatory cells and no microorganism visible.
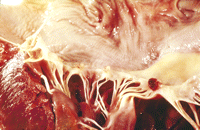

Large NBTE vegetations seen on the mitral valve leaflet. Only rarely are NBTE vegetations as extensive as these.
Allen and Sirota [6] proposed a macroscopic classification of NBTE (Table 2 ). In three large autopsy series [3,38,39], approximately 75% of vegetations were less than 3 mm in the largest diameter and 70% were multi-verrucal.
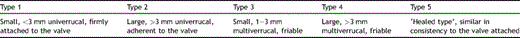
6 Management of NBTE
Even with an established diagnosis, treatment of NBTE is difficult. Correction of the underlying cause is of paramount importance. In patients with advanced and non-curable cancers, surgery is unlikely to influence the final outcome and also not prevent recurrent embolisation. In patients with potentially curable cancer, coagulopathy should be corrected and a multidisciplinary approach regarding the priority of surgery should be considered. If there is no contraindication, these patients should be anticoagulated with heparin [40] although there are no prospective randomised studies to support this strategy. Lee et al. [41], in a recent study of cancer patients with recurrent venous thromboembolism, compared low molecular weight heparin (LMWH) and oral anticoagulation. They found patients on LMWH to have less recurrent venous thromboembolism, and the probability of having a recurrent thromboembolism at 6 months to be reduced compared to oral anticoagulation. However, heparin induced thrombocytopenia may develop during therapy, as this can complicate the situation. In patients with APS and other autoimmune disorders presenting with recurrent cerebral embolism, the merit of surgical intervention needs to be tailored to the clinical presentation of each patient.
There are no guidelines for surgical intervention in patients with NBTE. If the patient is in acute congestive cardiac failure (due to valvular dysfunction) or occurrence of recurrent thromboembolism despite therapeutic anticoagulation then surgical intervention is warranted provided the complications and comorbid conditions does not make the prospect of recovery remote. In haemodynamically stable patients the decision for surgical intervention is difficult and should be avoided whenever possible.
7 Conclusion
NBTE is a not a common entity, but is frequently underestimated, probably due to the underlying disease (cancer, autoimmune disorders, HIV), and is difficult to diagnose and relies on strong clinical suspicion. It is difficult to manage and each case should be individually managed by identifying and treating the underlying pathology. Surgical intervention is not recommended unless the patient is in acute congestive failure.
Acknowledgement
We are very grateful to Dr P.J. Gallagher for his input with the histological images.




