-
PDF
- Split View
-
Views
-
Cite
Cite
Caroline Van De Wauwer, Dirk Van Raemdonck, Geert M. Verleden, Lieven Dupont, Paul De Leyn, Willy Coosemans, Philippe Nafteux, Toni Lerut, Risk factors for airway complications within the first year after lung transplantation, European Journal of Cardio-Thoracic Surgery, Volume 31, Issue 4, April 2007, Pages 703–710, https://doi.org/10.1016/j.ejcts.2007.01.025
Close - Share Icon Share
Abstract
Objective: Lung transplantation (LTx) has enjoyed increasing success with better survival in recent years. Nevertheless, airway anastomotic complications (AC) are still a potential cause of early morbidity and mortality. In this retrospective cohort study we looked at possible predictors of AC within the first year after LTx. Methods: Between July 1991 and December 2004, 232 consecutive single (n = 102) and bilateral (n = 130) LTx were performed (142 males and 90 females; mean age: 48 years [range 15–66 years]). Indications for LTx were emphysema (n = 113), pulmonary fibrosis (n = 45), cystic fibrosis (n = 35), pulmonary hypertension (n = 10), sarcoidosis (n = 7) and miscellaneous (n = 22). Donor variables (age, gender, PaO2/FiO2, mechanical ventilation, ischemic time and preservation solution) and recipient variables (age, diagnosis, length, gender, pre-operative steroids, smoking, cytomegalovirus matching, LTx type, anastomotic type, wrapping and bypass) were evaluated in an univariate and multivariate model. Results: Fifty-seven complications occurred in 362 airway anastomoses (15.7%) of which 55 (15.2%) within the first year after transplantation. Six patients died as a result of AC (mortality 2.6%) during the first year after LTx. In a univariate analysis (321 airway anastomoses at risk), anastomotic type (7/17 [Telescoping] vs 48/304 [End-to-end]; p = 0.011), recipient length (p = 0.0012), donor ventilation (>50–70 h≪; p = 0.0015) and recipient male gender (43/191 [M] vs 12/130 [F]; p = 0.0092) were significant predictors of AC. Three factors remained significant predictors in the multivariate analysis: telescoping technique (p = 0.0495), recipient length (p = 0.0029) and donor ventilation (p = 0.003). Conclusions: Tall recipients and those receiving lungs from donors with prolonged ventilation have an increased risk to develop bronchial anastomotic problems. An end-to-end anastomosis should be preferred. Airway complications remain a matter of concern after lung transplantation.
1 Introduction
In the early years following the first clinical attempt of lung transplantation (LTx) by James Hardy in 1963, bronchial complications, mainly necrosis and dehiscence, were the most frequent cause of morbidity and mortality in those patients who survived the first two post-operative weeks [1]. This is partly explained by the fact that the lung is the only organ where the arterial systemic blood supply is not restored routinely at the time of transplantation. Reestablishment of the bronchial arterial circulation can take up to 4 weeks [2,3]. The viability of the donor bronchus during that interval depends on the retrograde blood flow from the pulmonary circulation through collaterals [4,5].
In larger series, the incidence of airway complications (AC) after transplantation ranges from 7 to 14% of the anastomoses at risk with a related mortality of 2–3% [5]. It is believed that improvements in organ preservation, surgical technique, post-operative management and immunosuppression with better control of early and late allograft rejection have all resulted in a decreased incidence of airway anastomotic problems over the last decade [6,7].
The purpose of this retrospective study was to review the results of bronchial healing in our LTx population over the past 13 years and to determine factors that might be associated with impaired bronchial healing within the first year after lung transplantation.
2 Patients and methods
2.1 Study group
Between July 1991 and December 2004, a total of 232 isolated lung transplantations were performed, including 102 single lung transplants (SLTx) and 130 double lung transplants (DLTx). SLTx was performed in 65 male and 37 female patients and DLTx in 77 male and 53 female patients. Emphysema (n = 113) was the most common indication for lung transplantation followed by pulmonary fibrosis (n = 45), cystic fibrosis (n = 35), pulmonary hypertension (n = 10), sarcoidosis (n = 7) and miscellaneous (n = 22).
2.2 Donors
There were 132 male and 100 female donors with a mean age of 36 ± 1 years. Brain death was the result of a head injury in 124 donors, a spontaneous intracranial bleeding in 89, asphyxia in 7, ischemia in 6, hypoxia in 2, intracerebral tumor in 2, metabolic disorder and intoxication in 1 patient each. Donors were ventilated for 56 ± 3 h prior to organ retrieval. The mean partial oxygen pressure (PaO2) with a fraction of inspired oxygen (FiO2) of 1.0 and a positive end-expiratory pressure (PEEP) of 5 cm H2O was 496 ± 5 mmHg at the time of organ offer.
2.3 Operative technique
All lungs were recovered in the setting of multiple organ retrieval from heart-beating donors. After injection of prostaglandin (PGE1 (alprostadil, Prostin®, Pfizer) and PGI2 (epoprostenol, Flolan®, GlaxoSmithKline)) directly in the pulmonary artery, donor lungs were flushed with modified Euro-Collins® (n = 78) or Perfadex® (n = 154) solution including the same prostaglandin. Prior to extraction, the lungs were inflated and the trachea was stapled.
In the recipients, a single lung or bilateral lung transplantation was performed according to the diagnosis. A clamshell incision was performed in 39 patients, a bilateral anterior thoracotomy in 86, a unilateral posterolateral thoracotomy in 101, a bilateral posterolateral thoracotomy in 2, a unilateral anterior thoracotomy in 1, a combined anterior and posterolateral thoracotomy in 2 and a sternotomy in 1 patient. After pneumonectomy, the implantation was done in the following order: (1) bronchus; (2) pulmonary artery and (3) left atrium. A telescoping anastomosis (T) was performed in 23 anastomoses in the first 18 transplants using a 3/0 non-resorbable monofilament running suture for the membranous portion and interrupted U-sutures for the cartilaginous portion inverting the first donor ring into the recipient bronchus. Thereafter, we switched to an end-to-end technique in 309 bronchial anastomoses using a 3/0 non-resorbable monofilament running suture for the membranous portion and simple interrupted sutures for the cartilaginous portion (IE). Finally, in the 30 last anastomoses in this series we changed to a continuous end-to-end technique where the cartilaginous portion also was closed with a running suture (CE). The bronchial anastomosis was covered with either peribronchial fat (n = 333), pericardial fat (n = 13), intercostal muscle (n = 10), omentum (n = 1) or a combination (n = 5).
Cardiopulmonary bypass (n = 89) was used in case of inability of the recipient to tolerate single lung ventilation during pneumonectomy or implantation, or in case of development of primary graft dysfunction after implantation of the first lung.
2.4 Antifungal therapy
At the start of our lung transplantation program anti-fungal therapy consisted of oral itraconazole or intra-venous amphotericin B at low doses. Over the last 12 years all patients received daily inhalation therapy with amphotericin B (Fungizone®, 2 × 5 mg/day) until discharge. Oral nystatin (Nystatine®, 4 × 5 ml/day) was given in addition to all patients during their hospital stay.
2.5 Bronchoscopic surveillance
Patients underwent routine bronchoscopy immediately after the transplantation, prior to extubation and before hospital discharge to assess the viability of the bronchial anastomoses. Additional bronchoscopic evaluations were performed on a clinical basis whenever infection, rejection or an airway problem was suspected. During follow-up routine bronchoscopy was performed after 3, 6 and 12 months and yearly thereafter or whenever indicated by clinical parameters such as a decline in FEV1 or dyspnea.
2.6 Variables
Data of 232 patients (362 bronchial anastomoses) were reviewed for AC within the first year after LTx. Thirty-one patients (41 bronchial anastomoses) died within the first year after LTx of causes unrelated to AC and were excluded from the risk analysis. The remaining 201 patients (321 anastomoses) who died as a result of AC or with a minimum follow-up of 1 year were included in the univariate and multivariate analysis. Donor variables (age, gender, PaO2/FiO2, mechanical ventilation, ischemic time and preservation solution) and recipient variables (age, diagnosis, length, gender, pre-operative steroids, smoking, cytomegalovirus (CMV) matching, LTx type, anastomotic type [T vs IE + CE], wrapping and cardiopulmonary bypass) were evaluated.
2.7 Bronchial complications
For the purpose of this study, AC were defined as anastomotic dehiscence (partial or total), fistula (bronchovascular, bronchopleural or bronchomediastinal), anastomotic or lower airway stenosis or bronchomalacia that required surgical intervention, dilatation, stent implantation, laser therapy or endoscopic follow up. Bronchoscopy and CT scan were the most important diagnostic tools to define the complication.
2.8 Statistical analysis
Logistic regression models have been used to verify the relation between the presence of an airway complication within the first year and a set of predictors (for each predictor separately). To correct for the clustered structure in the data (possibly two lungs per patient), generalized estimating equations (GEE) are used. A multiple regression model has been build to determine the odds ratios and the 95% confidence interval (CI) for different predictors. A p-value of ≪0.05 was considered as significant.
All analyses have been performed with the statistical package SAS (version 9.1) using the GENMOD procedure to fit the GEE models.
3 Results
3.1 Incidence
A total 362 airway anastomoses were performed in 232 patients. Fifty-seven AC were diagnosed (15.7%) in 44 patients of which 55 (15.2%) occurred in 43 patients within the first year after transplantation including 33 men and 10 women with a mean age of 46 ± 2 years (range 20–66 years). Two AC (bronchomalacia: n = 1, stenosis: n = 1) developed later than 1 year and were excluded from the study. Partial or total airway dehiscences (n = 29) were diagnosed in 24 patients at 30 ± 4 days after transplantation. Significant stenosis (n = 14) developed in 11 patients at 75 ± 18 days after transplantation. No distal airway stenosis was diagnosed. In eight patients, a fistula occurred at 87 ± 33 days after transplantation. Finally, bronchomalacia (n = 4) occurred in three patients and time to diagnosis was 103 ± 17 days. In three patients with a DLTx, two different AC on each side were diagnosed.
During the first 9 years of our transplant program (from 1991 to 1999 with 8 transplants/year on average) 17 AC occurred in 103 airway anastomoses (16.5%). In the following years (from 2000 to 2004 with 31 transplants/year on average) this incidence slightly decreased to 14.7% (38 AC in 259 airway anastomoses) but the difference was not significant (p = 0.74). There was also no significant difference in type of AC between both time periods (Table 1 ).
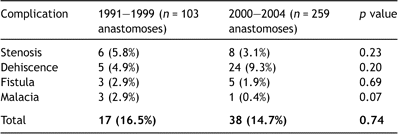
Comparison of airway complications between early (1991–1999) and late (2000–2004) experience
3.2 Morbidity and mortality
Six patients (2.6%) succumbed in hospital as a result of AC on post-operative days 25, 31, 47, 55, 144 and 280. Four patients exsanguinated from a bronchovascular fistula. In one patient postmortem examination showed the presence of Aspergillus. Two patients died of brain death resulting from prolonged hypoxemia. Histological evidence of fungal infection was provided after autopsy in one patient with a bronchomediastinal fistula. This patient died from sepsis not related to the airway complication.
In 17 patients traces of fungi were found during bacteriological examination of the broncho-alveolar-lavage that was taken at the time when airway complication was diagnosed.
Duration of post-operative mechanical ventilation and length of ICU stay were longer in patients with AC compared to those without but the difference did not reach statistical significance (12 ± 3 days vs 9 ± 1 days, p = 0.96 and 23 ± 3 days vs 18 ± 1 days, p = 0.13, respectively).
Hospital stay was significantly longer in patients with AC compared to those without (51 ± 5 days vs 41 ± 2 days, p = 0.012).
3.3 Treatment
Operative reintervention was necessary for nine anastomoses (2.5%) in eight patients. Four bronchial dehiscences and three fistulas were successfully covered with an intercostal muscle bundle. In one patient with a dehiscence an omentoplasty was used. Transplantectomy was necessary in one patient after bilateral LTx. Twenty-five anastomoses (6.9%) were treated by stent placement, laser debridement, balloon dilatation or a combination. Twenty-one anastomoses (5.8%) healed without intervention.
3.4 Risk factors
3.4.1 Univariate analysis
Donor variables are shown in Table 2 . Airway complications occurred more often in patients who received lungs from male donors (39/177) compared to lungs from female donors (16/144) (p = 0.034). The odds ratio equals 2.261 (CI: 1.204–4.244). No correlation was found between the number of AC and donor age, ischemic time, PaO2/FiO2 and preservation solution. There was a significant relation between the duration of mechanical ventilation in the donor and the presence of an AC (p ≪ 0.0015). The probability of developing AC was the highest for donors on mechanical ventilation between 50 and 70 h before organ recovery (Fig. 1 ).
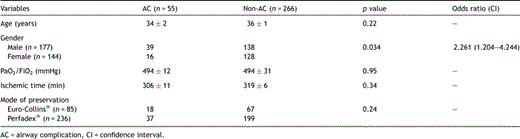
Donor variables for airway anastomotic complications (univariate analysis)
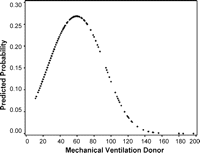
Relation between mechanical ventilation in the donor and the probability of an airway complication within the first year after lung transplantation. The probability is the highest in donors ventilated between 50 and 70 h prior to organ retrieval.
Recipient risk factors are summarized in Table 3 . There was a significant relation between the gender of the recipient and the development of an AC within the first year after transplantation (p = 0.0092). Male recipients (43/191) were more prone to AC than female recipients (12/130). The odds ratio equals 2.857 (CI: 1.297–6.295). The use of a telescoping technique (7/17) was associated with significantly more complications than an end-to-end anastomosis (48/304) (p = 0.011). The odds ratio equals 3.733 (CI: 1.119–12.457). The length of the recipient was also a significant risk factor for AC within the first year after transplantation (174 ± 1 cm in patients with AC vs 168 ± 1 cm without AC; p = 0.0012). The odds ratio for the effect of length (1 cm increase) equals 1.068 (CI: 1.026–1.112). Recipient age, diagnosis, pre-operative steroids, pre-operative smoking, type of operation, wrapping of the bronchial anastomosis, use of cardiopulmonary bypass and CMV matching were not related to AC.

Recipient variables for airway anastomotic complications (univariate analysis)
3.4.2 Multivariate analysis
Predictors from the univariate analysis with a p-value smaller than 0.2 were considered in a first step. Due to a large overlap of gender of donor and gender of recipient, only gender of recipient has been used in multiple models. In the final model mechanical ventilation in the donor, the use of a telescoping technique and length of the recipient remained significant risk factors (Table 4 ). Gender of the recipient was no longer a significant risk factor in the multivariate analysis (p = 0.82). The length of mechanical ventilation in the donor was associated with an increased risk for AC (p = 0.003). To describe this relation a quadratic term was needed (linear term OR: 1.114, CI: 0.765–1.623, quadratic term OR: 0.986, CI: 0.977–0.995). A figure is more informative to interpret the effect of mechanical ventilation in the donor than the estimated odds ratio. The probability to develop an AC was higher for donors on mechanical ventilation between 50 and 70 h before organ recovery (Fig. 2 ). The use of a telescoping anastomotic technique was associated with a 3.1-fold increased risk to develop AC (CI: 1.002–9.716; p = 0.0495). With the same duration of mechanical ventilation in the donor, recipients where a telescoping anastomotic technique was used had a higher probability to develop AC than patients where an end-to-end technique was performed (Fig. 2). Finally, the length of the recipient increased the risk with 1.1 (CI: 1.022–1.110; p = 0.0029).

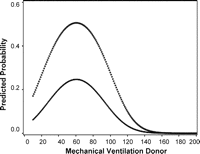
Relation between mechanical ventilation of the donor and the probability of a complication within the first year after lung transplantation for a patient of average length (170 cm). The relation is plotted separately for telescoping (open squares) and end-to-end (black dots) anastomotic technique.
4 Discussion
In this study the incidence of AC was 15.2% with a mortality of 2.6%. Duration of mechanical ventilation in the donor, recipients’ length and telescoping technique were identified in a multivariate analysis as risk factors to develop AC within the first year after transplantation.
The incidence of AC in our study is similar to that reported in the literature. However, adequate comparison of the incidence of AC between different transplant centers is limited by a number of factors. First, there is no internationally accepted classification of AC after lung transplantation. Currently, two grading systems are proposed. The classification by Couraud is based on bronchoscopic staging of early anastomotic healing. In the classification by Shennib and Massard early but also late AC were included [5]. Secondly, in several studies, only AC that necessitated surgical or medical intervention, were selected to calculate the incidence. In our study, we included all AC diagnosed on bronchoscopic evaluation. But in only 2.5% operative reintervention and in 6.9% a medical intervention was necessary. In the remaining 5.8%, healing was successful with no further treatment. Thirdly, the surgical technique used for the bronchial anastomosis differs widely amongst previously reported series [8]. Several transplant centers used an end-to-end technique with a running suture for the membranous part and interrupted sutures for the cartilaginous part while others used a telescoping technique or a mixture of both.
To our knowledge, this study is the first to associate AC with a long (>50–70 h≪) mechanical ventilation in the donor. Mechanical ventilation for >48 h is well known risk factor for ventilatory associated pneumonia in 10–20% of the patients [9]. In previous studies, pre-operative and post-operative infections in the lung were defined as risk factors for AC [10,11]. It is also known that organisms present in the donor lung have an influence on the development of post-operative infection in the recipient. Investigators have reported a strong correlation between the presence of Aspergillus, bronchial wall necrosis and occurrence of AC [11]. The role of post-operative fungal infection was also demonstrated in a recent report on AC after pediatric lung transplantation [10]. Pre-operative infection with Pseudomonas cepacia and duration of post-operative mechanical ventilation were defined as risk factors in the same study. We hypothesize that longer ventilation in our donor population may be associated with an increased risk for pulmonary infection in the recipient and subsequent post-operative AC but unfortunately the infection rate in our LTx population was not reviewed in this study.
At the beginning of our lung transplant program in 1991, the telescoping technique was used and this was associated with an unacceptable high incidence of AC (7/17 or 41%). The routine use of this technique was therefore rapidly abandoned in favor of the end-to-end anastomosis [12]. Garfein and co-workers also reported a higher incidence of AC associated with the telescoping technique in patients who underwent single lung transplantation for emphysema [8]. In contrast with this, other investigators observed good results [13] with telescoped anastomoses and no correlation was found between telescoped anastomoses and the development of an AC [14,15]. The small number of telescoped anastomoses in comparison with the end-to-end technique in this series could be a potential limitation in the comparison of both techniques. In a recent study, the use of a single running suture provided excellent results with a very low complication rate [7]. Currently, we are also using an end-to-end technique with a continuous running suture on the membranous and cartilaginous part with an absorbable 4/0 monofilament. We have been very satisfied with this technique so far that has lead to a decrease of AC in our recent series.
We also found that the length of the recipient was a risk factor for AC. In 31 out of 55 bronchial anastomotic complications, emphysema was the indication for transplantation. In the group that developed AC, patients with emphysema were taller than non-emphysematous patients although this difference was not significant. Tall patients have a larger circumference of the bronchus and emphysema patients usually present with less peribronchial fatty tissue that can be used to cover the anastomosis. We speculate that these factors may explain the increased risk for AC in these patients.
A discrepancy in length and thus in airway diameter between the donor and the recipient may play a role in the development of airway complications. As a result of routine size matching at the time of organ offer, there was a significant positive correlation between donor and recipient length in both groups with (p ≪ 0.0001, r = 0.75) and without (p ≪ 0.0001, r = 0.70) AC (Fig. 3a and b , respectively). Therefore we do not believe that size discrepancy in our study was a contributing risk factor in the development of AC.
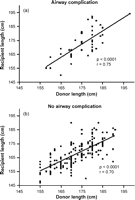
(a) Correlation between the length of the donor and the length of the recipient in patients with an airway complication. (b) Correlation between the length of the donor and the length of the recipient in patients without an airway complication.
Our study has several limitations. First, some early, asymptomatic AC may have been missed in the interval between two bronchoscopies. This seems very unlikely because three routine bronchoscopies were performed in the post-operative period before discharge. Second, the study was retrospective and the experience increased over time with more transplantations performed each year. Patients were not randomly assigned to a specific surgical technique of airway anastomoses. Therefore only historical comparison between different anastomotic techniques was possible. So the high incidence in AC with the telescoping technique may be related to our early experience with few numbers of transplants performed annually. Thirdly, transplantations were performed by five different surgeons. Nevertheless, the anastomotic technique in a certain time period of the study did not differ between surgeons. However, we did not include the surgeon as a variable in the risk analysis.
AC have been a major concern since the start of lung transplantation in the late sixties [1]. Bronchial healing is impaired by the absence of the direct bronchial blood supply during the first weeks after transplantation. The viability of the donor bronchus during that time depends on the retrograde blood flow from the pulmonary circulation through collaterals. Several ways to decrease the incidence of AC have been explored. The routine use of an omental wrap to protect the anastomosis has led to the first successful lung transplantations [16]. Nevertheless, the disadvantages related to this technique have stimulated surgeons to use other protective flaps such as pericardial, peribronchial or intercostal tissue [5]. However, the need for routine covering of the anastomosis is still debated. In a randomized clinical trial by Yacoub and co-workers, the incidence of bronchial anastomotic complications after single lung transplantation was not affected by wrapping the anastomosis with either omentum or an internal mammary artery pedicle [17]. Normal bronchial healing was achieved without wrapping in a canine single lung transplant study [18]. Previous studies have demonstrated that covering the anastomosis has a positive influence on the microcirculation as revascularization of the anastomosis is possible via collateral circulation from the wrap [17,19]. Other surgical improvements in recent years may also contribute to better healing of the anastomosis. Preservation of peribronchial tissue and division of the donor bronchus one ring proximal to the orifice of the upper lobe bronchus reducing the segment that depends on retrograde pulmonary blood flow have been suggested as important surgical steps to reduce bronchial ischemia [20].
Revascularization of the bronchus can improve airway healing. An anatomical study of the bronchial arteries in autopsy cases showed that at least one right bronchial artery arises from the first intercostal artery and that at least one left bronchial artery originates from the descending thoracic aorta [21]. Injection studies of the right intercostobronchial artery pedicle showed a collateral network between the right and the left bronchial arteries. Direct bronchial artery revascularization has been attempted by some centers and proved to be possible in en bloc double lung transplantation [22,23] as well as in single lung transplantation [24]. The telescoping technique was introduced into clinical practice to enforce the bronchial anastomosis with initially good results [13]. However, other data suggest that intussusception of the donor bronchus in the recipient bronchus may promote the development of late stenosis [25].
Prostaglandins have been advocated as a beneficial additive to the preservation solution because it improves graft microcirculation by means of vasodilatation with a more rapid and effective distribution of the preservation solution as a result. Increased bronchopulmonary collateral blood flow with improved bronchial perfusion has been demonstrated after administration of prostacyclin to the donor prior to the pulmonary flush and to the recipient during reperfusion [26]. In our study all donor lungs were preserved using prostaglandins.
Despite these surgical achievements, AC continues to be a cause of morbidity and a risk for early mortality. Therefore, critical analysis of potential risk factors that may play a role in the development of AC is important. Date and co-workers from St. Louis [14] demonstrated that single lung transplantation and the use of mattress suture were associated with a higher prevalence of AC. This group also suggested that better maintenance immunosuppression and rejection surveillance reduced the prevalence of AC. In our study, however, there were more complications after bilateral lung transplantation compared to single lung transplantation although the difference did not reach statistical significance.
In a recent report by Ruttmann and co-workers [27] severe reperfusion edema with subsequent longer ventilation time and greater positive end-expiratory pressure, rejection within the first post-operative month, the use of interleukin-2 receptor antibody and interrupted suture technique of the anterior wall were associated with bronchial complications. Reperfusion edema and rejection within the first month remained significant in multivariate analysis. Unfortunately, in our study we were not able to look up data on reperfusion edema for the whole cohort because of missing chest X-rays necessary to grade primary graft dysfunction conform the new ISHLT classification. In other studies [14,27,28] AC also resulted in longer intubation periods post-operatively. In our study, we also found a longer duration of mechanical ventilation in patients with AC although the difference did not reach statistical significance.
In conclusion, we have identified duration of mechanical ventilation in the donor, length of the recipient and the use of telescoping technique as risk factors to develop airway complications within the first year after transplantation. Airway anastomotic complications remain an important matter of concern because of early associated morbidity and mortality after lung transplantation.
Presented at the joint 20th Annual Meeting of the European Association for Cardio-thoracic Surgery and the 14th Annual Meeting of the European Society of Thoracic Surgeons, Stockholm, Sweden, September 10–13, 2006.
Acknowledgement
The authors would like to thank Steffen Fieuws for his help with the statistical analysis.
Appendix A
Conference discussion
Dr W. Weder (Zurich, Switzerland): Twenty years ago, the complication of bronchial anastomosis seemed to be the Achilles’ heel for the development of lung transplantation. You have shown that the risk now for developing a complication is low, but still there are problems.
I have a question regarding your observation on the dependence of bronchial anastomotic complications from gender. We know that the risk of rejection of the lungs is also depending from the donor sex. Organs from female donors are less likely rejected. Is your observation related also to long-term rejections or is it a specific immunological factor? Did you look at that, or what is your explanation for this gender influence?
Dr Van De Wauwer: We did not correlate the gender of the donor or the gender of the recipient with the risk of rejection.
Dr A. Haverich (Hannover, Germany): I’m just curious whether the length of intubation and ventilation of the recipient play a significant role, because this is our impression from the data we have in Hannover.
Also, the question of warm ischemic time, which is longer in the double-lung or bilateral-lung transplantation than in the single lung transplantation, doesn’t that play a role in your series?
Dr Van De Wauwer: In answering your first question, we also looked at a possible difference in time of intubation after the lung transplantation, but there was no significant difference between both groups, although the intubation time was longer in the patients who developed an airway complication compared to the patients who did not have an airway complication.
In answering your second question, we also compared the ischemic time and there was no significant difference in ischemic time between the group where an airway complication developed compared to the group without airway complication.
Dr W. Klepetko (Vienna, Austria): I wonder whether the length of the mechanical ventilation of the donor really has an impact on the whole thing or it might be rather factors which are inherent to the length of intubation, for instance potential infection within a donor lung. In addition, your very aggressive use of marginal donor lungs now, should have brought you some insight whether this might also have an impact on the healing of the bronchial anastomosis due to a higher frequency of infections.
Particularly, I would like to know your regimen for antifungal prophylaxis after transplantation and whether these four cases of bronchovascular fistula you reported, were related to fungal infections and when they did occur?
Dr Van De Wauwer: Our hypothesis is that the association between the development of airway complications and the ventilation in the donor was related to the increased risk in those donors to develop pneumonia and a subsequent higher risk of airway complications in the recipients of those lungs.
In answer to your second remark, the bronchovascular fistulas developed at several time points after the operation. A few fistulas developed quickly after the lung transplantation was performed, but in one patient the bronchovascular fistula developed almost 1 year after the lung transplantation.
In answer to your question about the antifungal therapy, I can’t really give you that answer because at this moment I’m working in the laboratory and I’m not familiar with the therapy that's given in the hospital.
Dr H. Bittner (Leipzig, Germany): Your group is to be congratulated on how to manage and how to use marginal donor organs successfully. My question therefore, is, whether some of those marginal donor organs were included in your assessment? And the second question relates to the technical aspects of performing bronchial anastomoses. When you are performing a telescoping bronchial anastomosis, was the donor bronchus primarily intubated into the recipient or vice versa? When you invaginate the donor into the recipient a rim establishes which, exposed to long-term ventilation and high volume flows and pressure, may lead to dehiscence and necrosis.
Dr Van De Wauwer: In this series there were no marginal donors included. The telescoping technique was only used in the first lung transplantations. We performed an invagination of the donor bronchus into the recipient bronchus and in those cases there was no longer mechanical ventilation.
Dr N. Yonan (Manchester, United Kingdom): I’m sure we all agree that it's related to ischemia and the early phase of development of dehiscence or stenosis as a later consequence. Did you look in the double-lung group? Did you look at which lung, if you have both sides, which side, left or right, was affected? Is it equally or one side more than the other? Did you compare the ischemic time in those patients with the development? Because the feeling is it is definitely related to ischemia.
Dr Van De Wauwer: We made only a difference between bilateral lung transplantation and single-lung transplantation and there was no significant difference. But we did not note any difference between the right lung or the left lung.




