-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher M. Burton, Martin Iversen, Nils Milman, Mikhail Zemtsovski, Jørn Carlsen, Daniel Steinbrüchel, Jann Mortensen, Claus B. Andersen, Outcome of lung transplanted patients with primary graft dysfunction, European Journal of Cardio-Thoracic Surgery, Volume 31, Issue 1, January 2007, Pages 75–82, https://doi.org/10.1016/j.ejcts.2006.10.024
Close - Share Icon Share
Abstract
Objective: Primary graft dysfunction (PGD) causes significant mortality and morbidity after lung transplantation. The objectives of the study were to describe the clinical and histological sequelae of PGD. Methods: Histology of all patients receiving single-lung transplantation 1999–2004 (n = 181) was reviewed. PGD was defined as diffuse radiological infiltration of the lung allograft occurring within the first 72 h postoperatively. Results: One patient died intra-operatively. PGD was recorded in 63% (n = 113) of 180 consecutive transplant recipients. Patients with PGD had a worse 90-day postoperative mortality (14% versus 3%, p = 0.03) and 3-year survival (55% versus 77%, p = 0.003). Freedom from bronchiolitis obliterans syndrome was similar in both groups. The maximal FEV1 was significantly lower in patients with PGD, median 54% (quartiles 48–61%) predicted; compared to patients without PGD, median 59% (quartiles 54–69%) predicted (p = 0.003). There was a significant linear trend in the decline of maximal FEV1 with the presence and increasing severity of radiographic infiltrate (p = 0.004). During follow-up, patients with PGD were more likely to demonstrate diffuse alveolar damage or bronchiolitis obliterans organizing pneumonia (p = 0.009 and p = 0.01, respectively). Histological findings of diffuse alveolar damage correlated closely with extent of radiological infiltration (p ≪ 0.0001). Conclusions: Transplant recipient survival, lung function, and histological findings of diffuse alveolar damage appear to be closely correlated with the appearance and severity of PGD.
1 Introduction
A proportion of lung-transplanted patients develop early diffuse infiltrates visible on chest radiographs and delayed graft function which may represent reperfusion edema [1]. Reperfusion edema is believed to be a non-immune mediated inflammatory process which is clinically analogous to adult respiratory distress syndrome (ARDS) in the most severe cases [2]. Reperfusion edema has not only been associated with a high perioperative mortality [3–5], but also with poorer long-term survival [6] and more rapid progression to bronchiolitis obliterans syndrome (BOS) [7], than in patients without clinical evidence of reperfusion edema.
To co-ordinate future research and facilitate the exchange of data, the International Society of Heart and Lung Transplantation (ISHLT) Working Group on Primary Lung Graft Dysfunction has recently defined and graded reperfusion edema as primary graft dysfunction (PGD) based on radiological appearances and the PaO2:FiO2 ratio [8]. Although ARDS is manifest as a pattern of diffuse alveolar damage (DAD) histologically, the ISHLT consensus report does not incorporate histological findings into the definition.
The objectives of the present study were to describe the outcome of these patients in relation to survival, long-term lung function, and histological findings.
2 Materials and methods
2.1 Subjects
All single-lung transplanted (SLTX) patients from 1999 to 2004 (n = 181) were included in the study. Digital imaging and database software for all radiology first became available in 1999 at this center. Analysis of chest radiographs was restricted to this period in order to minimize the risk of patient misrepresentation, and permit all radiographs to be assessed under optimal viewing conditions. Only patients receiving SLTX were included in the study on the pretense that it would be easier to grade chest radiographs with respect to the presence and extent of edema and consolidation, by comparing changes relative to the patients’ native lung. All chest radiographs pertaining to the first 72 postoperative hours (including a preoperative radiograph for comparison) were reviewed in chronological order by two expert lung transplant physicians, independently. The reviewers were blinded to patient identification variables, additional forms of imaging (such as computerized tomography (CT) scans), and to imaging obtained later than 72 h postoperatively. Additional demographic and clinical data were obtained by retrospective chart review. PGD was diagnosed if both reviewers agreed on the presence of a unilateral diffuse radiological infiltrate of the lung allograft. Patients with PGD were sub-classified according to (a) the lowest recorded PaO2:FiO2 ratio, and (b) the inter-reviewer agreement of the maximal extent of radiological infiltration (Fig. 1 ).
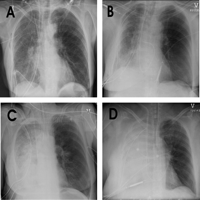
Four examples of chest radiographs with diffuse infiltrates classified as (A) minimal—diffuse infiltrate only, (B) mild—diffuse infiltrate with areas of consolidation (≪50% parenchymal involvement), (C) moderate—diffuse infiltrate with consolidation (≥50% but less than 90% parenchymal involvement), and (D) severe—≥90% consolidation. An ‘infiltrate’ refers to opacity with visible underlying parenchymal structure. The term ‘consolidation’ refers to opacities whereby the underlying parenchymal structure could not be visualized. Accentuation of hilar structures (seen in over 90% of early radiographs) was not considered in this radiographic classification.
2.2 Transplantation procedure
The transplantation procedure, and donor and recipient selection and management have been described previously [9]. In brief, procured lungs were irrigated with 4 l modified Eurocollins® solution with added prostacyclin (PGI2). The lungs were inflated to 60% of the TLC with 40% FiO2, and maintained at approximately 4 °C. SLTX generally involved a posterolateral thoracotomy, resection of the recipient’s native lung and implantation of the donor lung without the need for routine institution of cardiopulmonary bypass. On-table extubation was performed if patients had a satisfactory conscious level (Glasgow Coma Score ≥12), body temperature >36 °C, stable hemodynamic condition without inotropic medication, PaO2 >10 kPa, PaCO2 ≪8 kPa, FiO2 ≪40%, and acceptable post-operative hemorrhage [10]. All transplanted patients received induction therapy with either antithymocyte globulin (ATG) or daclizumab (an IgG1 monoclonal antibody for interleukin-2 receptor). Thereafter, patients received triple maintenance immunosuppressive therapy consisting of cyclosporine, azathioprine and prednisolone. Blood cyclosporine concentration was maintained in the range 145–245 μg/l. Acute rejection (≥A2) episodes were treated with intravenous methylprednisolone 1 g daily for 3 days, followed by prednisolone tapered to maintenance dose over a 3-week period.
2.3 Histological surveillance
Protocol flexible bronchoscopy surveillance with routine transbronchial biopsy (TBB) and broncho-alveolar lavage (BAL) was performed in all patients at 2, 4, 6, 12, 26, 52, 78 and 104 weeks. Three biopsies consisting predominantly of alveolar tissue were considered sufficient for histological evaluation, but in most cases at least five biopsies were obtained. Histological grading of all allograft material with respect to acute cellular infiltrate, lymphocytic bronchiolitis, bronchiolitis obliterans, and vasculitis was performed by a single pathologist according to the standard criteria [11]. The presence of additional inflammatory interstitial patterns such as DAD, bronchiolitis obliterans organizing pneumonia (BOOP), and interstitial pneumonitis were also noted [12]. Patients with histological evidence of diffuse alveolar damage, bronchiolitis obliterans organizing pneumonia, or obstructive pneumonitis were re-classified as a single group with histological features of organizing pneumonia.
2.4 Statistical analysis
For the analysis of histological data, the time from transplantation for all transbronchial biopsies was calculated for each patient. These calculated time intervals were subsequently grouped together according to the closest of the scheduled surveillance windows (for example, if a patient had biopsies taken at 3.5 and 4.9 weeks, both biopsies would be grouped in the 4 weeks surveillance window). If, as in the aforementioned example, two or more transbronchial biopsies were performed within the same surveillance window, the most severe histological grade of acute cellular rejection was recorded for analysis.
Forced expiratory volume in 1 s (FEV1) values obtained within the 3 months prior to death were excluded from calculation of the BOS grade. The BOS grade was otherwise calculated in accordance with the criteria proposed by Estenne et al. [13]. FEV1 and forced vital capacity (FVC) values were presented as percent predicted which were calculated using formulas published by the European Respiratory Society [14].
Data analyses were performed using Statistical Analysis Software (SAS®) version 9.1 and Statistical Package for the Social Sciences (SPSS®) 11.2. Continuous data are described as mean ± standard deviation (±SD) or median and quartiles for normal and skewed distributions, respectively. Two group comparisons were performed by Student’s t-test or Mann–Whitney test, as appropriate. Comparisons between categorical data were performed by Chi square test. Holm’s correction method was employed for multiple pair-wise comparisons. Survival data was assessed by Kaplan–Meier method, and group comparisons were made by log rank test. Hazard ratios (HR) are given with 5% and 95% confidence intervals (CI).
3 Results
3.1 Patients
The total number of SLTX recipients in the study period between February 1999 and December 2004 was 181. One male patient with a pre-transplant diagnosis of idiopathic pulmonary fibrosis died due to intra-operative cardiac arrest, and was excluded from subsequent analyses. Thus 180 patients were available for radiographic assessment and subsequent analysis.
PGD as defined by the presence of diffuse radiological infiltrate was recorded in 63% (n = 113) patients (Table 1 ). Inter-observer and intra-observer kappa coefficients for the presence or absence of infiltrates were 0.51 and 0.72, respectively. The inter-observer weighted kappa coefficient for the sub-classification of patients according to the severity of infiltrate was 0.49.
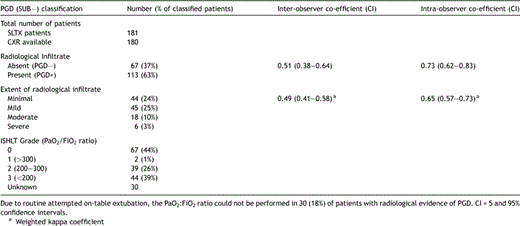
Distribution of patients with respect to the presence and severity of primary graft dysfunction (according to either the extent of radiological infiltration (see text) or the PaO2:FiO2 ratio) with respective inter- and intra-observer kappa coefficients
On-table extubation was performed in 102 (57%) patients, and therefore, determination of the PaO2:FiO2 ratio could not be performed in 30 (18%) of patients with radiological evidence of PGD.
The distribution of patients with and without PGD according to recipient and donor demographic, and operative and postoperative variables is shown in Table 2 .
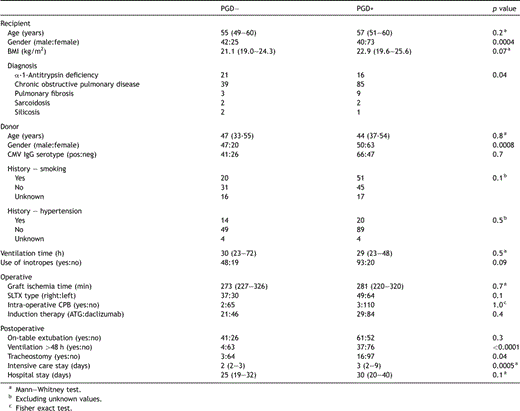
Selected recipient, donor and perioperative demographic and outcome variables according to the presence or absence of primary graft dysfunction
3.2 Prognosis
Post-operatively, the presence of PGD was associated with a significantly larger proportion of patients requiring >48 h ventilation and tracheostomy (p ≪ 0.0001 and p = 0.04, respectively) (Table 2), with a significant trend to increasing risk of prolonged ventilation and tracheostomy with increasing radiological severity (two-sided Cochran-Armitage trend test p ≪ 0.0001 and p = 0.0004, respectively). Patients with PGD also spent more time in intensive care; however, the total hospital stay was similar between the two groups (Table 2). Extra-corporeal membrane oxygenation (ECMO) was employed on two occasions; both patients had moderate-severe infiltrates.
Patients with PGD had a worse prognosis in terms of 90-day postoperative mortality (14% versus 3%, p = 0.03, HR = 5.0, CI 1.1–21.7) and 3-year survival (55% versus 77%, p = 0.003, HR = 2.5, CI 1.3–4.7), respectively (Fig. 2 ). There was a significant survival trend between patient subgroups according to the presence and extent of radiological infiltrate (p = 0.002), but not comparing ISHLT grades 0–3 according to the PaO2:FiO2 ratio (p = 0.07).
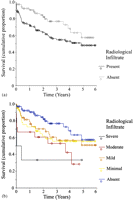
Kaplan–Meier plots of cumulative proportion of patients surviving, according to (a) the presence or primary graft dysfunction (p = 0.02); and (b) the extent of the radiological infiltration.
Baseline FEV1 and respective BOS grades were calculated from 3296 post-transplant spirometry measurements. There was no significant difference in the number, or time interval between, lung function measurements in patients according to the presence or absence of PGD. The maximal FEV1 was significantly lower in patients with PGD, 54% (48–61%) predicted; compared to patients without PGD, 59% (54–69%) predicted (p = 0.003). FVC was similar in patients with PGD, 67% (57–77%) predicted, and without PGD, 70% (64–79%) predicted (p = 0.1). Fig. 3 demonstrates the distribution of maximal FEV1 and FVC according to the presence and severity of radiographic infiltration. There was a significant linear trend in the decline of maximal FEV1 with the presence and increasing severity of radiographic infiltrate (p = 0.004).
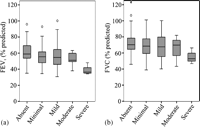
Maximum (a) forced expiratory volume in 1 s (FEV1) and (b) forced vital capacity (FVC) according to the presence and extent of radiological infiltration.
Patients surviving more than 3 months (n = 151, 84%) were included in a sub-analysis of freedom from BOS. The freedom from BOS grades 0–3 was similar in patients with and without PGD, and there were no demonstrable trends between severity of infiltrate or PaO2:FiO2 ratio, and progression to BOS grades 0p–3.
3.3 Histology
A total of 875 post-transplant transbronchial biopsy specimens pertaining to 65 (97%) PGD− and 110 (97%) PGD+ patients were available for analysis. Excluding patients with missing transbronchial biopsies, a mean number of 9 ± 3 and 8 ± 3 histological specimens were obtained from patients with and without PGD, respectively (p = 0.5). The median number of completed transbronchial biopsy surveillance time points (maximum of 8) was 6 in the PGD− group and 7 in the PGD+ group.
Fig. 4 demonstrates the cumulative proportion of patients free from acute cellular rejection (≥A2), organizing pneumonia, interstitial pneumonitis, and bronchiolitis obliterans according to presence or absence of PGD. During follow-up, PGD+ patients were more likely to develop histological evidence organizing pneumonia (p = 0.0004), comprising either DAD and/or BOOP (p = 0.009 and p = 0.01, respectively). The cumulative incidence of interstitial pneumonitis (which included non-specific, desquamative and unclassified types) was also higher in patients with PGD (p = 0.02), however, the cumulative incidence of acute cellular rejection was highest in the group without PGD (p = 0.07). The cumulative incidence of bronchiolitis obliterans was similar in both PGD groups (p = 0.3).
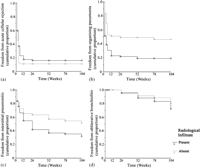
Kaplan–Meier plots of cumulative proportion of patients free from (a) acute cellular rejection (≥A2) (p = 0.07); (b) organizing pneumonia (p = 0.0004); (c) interstitial pneumonitis (including non-specific, desquamative and unspecified sub-types) (p = 0.02); and (d) bronchiolitis obliterans (BO) (p = 0.3) according to presence of primary graft dysfunction.
There was a strong correlation between the increasing severity of radiological infiltration and the histological findings of organizing pneumonia (p = 0.0003), comprising DAD (p ≪ 0.0001) and/or BOOP (p = 0.03) (Fig. 5 ).
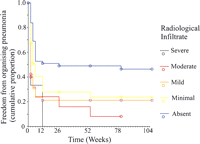
Kaplan–Meier plots of cumulative proportion of patients free from organizing pneumonia (trend test p = 0.0003), according to the presence and extent of radiological infiltration – refer to text.
4 Discussion
The ISHLT have recently published extensive guidelines covering the definition, potential risk factors, outcomes and possible treatments for PGD [8]. The guidelines define PGD as the occurrence of a diffuse radiological infiltrate of the lung allograft within the first 72 h after transplantation after excluding other causes of pulmonary edema (such as hyper-acute rejection, venous anastamotic obstruction, cardiogenic pulmonary edema, and pneumonia) which can subsequently be graded according to the worst recorded PaO2:FiO2 ratio. There is also provision to record the time of onset of radiological infiltrate. Histological findings, however, have not been incorporated into the consensus definition of PGD. As such, the ISHLT definition of PGD is essentially a radiological diagnosis with provision for sub-classification according to onset and associated arterial hypoxemia.
Employing the new ISHLT consensus definitions, we demonstrate that patients with PGD (1) experienced a longer duration intensive care stay, an increased risk of perioperative mortality and a lower 3-year survival, (2) had a lower baseline FEV1 after lung transplantation despite no change in the rate of decline in lung function, and (3) a higher cumulative incidence of organizing pneumonia (particularly DAD) on subsequent surveillance protocol transbronchial biopsy. In addition, the study demonstrates that grading PGD in terms of the extent of radiological infiltration rather than PaO2:FiO2 ratio was sufficient in illustrating trends between the severity of PGD and the decline in baseline FEV1, the increasing cumulative incidence of organizing pneumonia (particularly DAD), and worsening patient survival.
Prior to the new ISHLT consensus definition of PGD, the reported incidence of reperfusion edema varied widely. The highest incidences of reperfusion injury were reported in series employing solely radiological definitions [15]. Other series have employed both radiographic criteria in combination with measures of oxygenation [1,3,5,7,16]. Although Christie et al. [1] reported that all patients in their 1998 cohort had histological evidence of DAD, only the study by Fisher et al. in 2002 [17], defined PGD solely on the presence of this histological finding either by TBB at 7 days or by autopsy.
As the presence of PGD is now essentially a radiological diagnosis, it is not surprising that the finding of PGD in 63% of the SLTX recipient population at this center is similar to the incidence of reperfusion edema reported by other centers using only radiographic criteria [15]. The radiological findings of isolated peri-hilar infiltrate were not classified as PGD in this study. Indeed, peri-hilar infiltrate was a common finding as has been reported previously [18], and was identified in almost all SLTX recipients. The incidence of moderate to severe radiological infiltrate in our cohort of patients (13%) is similar to the incidence of PGD as defined by more stringent criteria by King et al. [5] and Christie et al. [3]. King et al. reported an incidence of 22% in 120 patients whereby PGD was defined as a severe radiological infiltrate in association with a PaO2:FiO2 ratio ≪200 during the first 48 h after transplantation. Using a more stringent definition whereby the PaO2:FiO2 ratio ≪200 should persist beyond 48 h after transplantation; Christie et al reported an overall PGD incidence of 11.8% in their study of 255 patients.
Despite the discrepancies in the definition of reperfusion injury, there is some agreement that these patients have a prolonged requirement for ventilatory support (and by inference, delayed discharge from intensive care) [1,4,5,15], and a higher post-operative mortality [4,5]. In our own series, PGD was documented as the primary cause of death in 41%, and a contributory cause of death in 18% of all lung transplanted patients not surviving until hospital discharge [9]. This is similar to the findings reported from an analysis of 5262 patients from the United Network for Organs Sharing (UNOS) registry whereby 43.6% of patients dying in the first 30 days had PGD [6]. The same study also showed that for patients surviving more than 1 year, patients with earlier PGD had significantly worse survival over ensuing years than for patients without PGD. The results of the present study corroborate with these earlier observations.
In the most severe cases PGD resembles ARDS, the histological hallmark of which is a pattern of DAD [1,17]. The results of the present study support a temporal association between clinical PGD and histological DAD. In addition, the findings support a correlation between the extent of radiographic infiltration and the cumulative incidence of DAD. There was also a significantly higher cumulative incidence of BOOP in the group of patients with PGD.
BOOP is usually identified in association with other medical conditions and rarely occurs in the idiopathic form [19], and has been reported in the context of lung, bone marrow, and other solid organ transplantation [20], and also in association with the use of immunosuppressive therapy with sirolimus and tacrolimus [21,22]. All patients in this study-received cyclosporine based triple immunosuppressive therapy, exclusively. We believe that early BOOP is likely to be the result of non-specific epithelial injury of multiple etiology.
We were unable to demonstrate a relationship between the development of BOS and earlier PGD in a sub-analysis of patients surviving more than 3 months. However, patients with PGD had a lower median maximal FEV1, which decreased with increasing radiographic severity. The median maximal FEV1 is approximately 39% lower in recipients with severe PGD compared to patients without PGD, which perhaps, would be sufficient to explain the observed effects on morbidity [4] and survival.
As with all retrospective studies, we are unable to exclude the possibility of bias. In addition, the inter- and intra-observer agreement was lower than anticipated; although, the higher intra-observer agreement suggests that training may improve consistency. With hindsight, radiographic sub-classification into four groups of severity based on the percentage of lung affected may have been too ambitious. Firstly, because of the introduction of human error in visually identifying such proportions; and secondly because more severely involved edematous parenchyma may shrink and appear as a smaller proportion of the whole lung when imaged by CXR. However, other underlying components of the proposed radiological sub-classification, such as the distinction between infiltration and consolidation may be a more important reflection of the severity of PGD.
We chose to study SLTX patients exclusively primarily in order to reduce potential misdiagnosis of PGD in double lung-transplanted patients with fluid overload. In addition, one would expect the patient cohort to be a more homogeneous in terms of age, preoperative functional NYHA status, and pre-transplant diagnosis; and that both PGD+ and PGD− groups would be subject to the same degree of confounding relating to intra- and post-operative donor organ stress. Indeed, this approach appears to have been validated in a recent study by Oto et al. [23] recommending that studies of PGD in DLTX and SLTX should be considered separately. In this study, possible artifacts related to earlier extubation and the effects of the native lung in SLTX recipients contributed to the apparently paradoxical outcomes of more severe PGD (graded according to the PaO2:FiO2 ratio) but shorter intubation requirements and a tendency to better short-term outcomes than was seen in the DLTX group. Certainly, extubation would be expected to reflect a less severe clinical manifestation in patients with PGD. In the present study, the grading of the severity of PGD based on the PaO2:FiO2 ratio was also problematic. Accurate determination of the PaO2:FiO2 ratio requires the patient to be on a sealed ventilatory system. Since 57% of the SLTX recipients were extubated in the operating room, a proportion of patients with PGD were not receiving a known FiO2. In non-ventilated patients receiving supplementary oxygen, several large assumptions would be required to estimate the FiO2, the true value of which is respiratory rate dependent.
Despite these limitations we were able to demonstrate the reproducibility and prognostic reliability of radiologically defined PGD in terms of early and late survival, and demonstrate a correlation between the extent of radiological infiltrate and histological findings consistent with organizing pneumonia, particularly DAD pattern.
5 Conclusions
The development of PGD was associated with a higher perioperative mortality and poorer long-term survival. Although the rate of loss of lung function (progression to BOS) is the same in both groups, baseline FEV1 was significantly lower in the group with PGD. Organizing pneumonia, particularly DAD, was the most closely correlated histological finding in patients with clinical PGD.




