-
PDF
- Split View
-
Views
-
Cite
Cite
Johan Sjögren, Malin Malmsjö, Ronny Gustafsson, Richard Ingemansson, Poststernotomy mediastinitis: a review of conventional surgical treatments, vacuum-assisted closure therapy and presentation of the Lund University Hospital mediastinitis algorithm, European Journal of Cardio-Thoracic Surgery, Volume 30, Issue 6, December 2006, Pages 898–905, https://doi.org/10.1016/j.ejcts.2006.09.020
Close - Share Icon Share
Summary
Poststernotomy mediastinitis, also commonly called deep sternal wound infection, is one of the most feared complications in patients undergoing cardiac surgery. The overall incidence of poststernotomy mediastinitis is relatively low, between 1% and 3%, however, this complication is associated with a significant mortality, usually reported to vary between 10% and 25%. At the present time, there is no general consensus regarding the appropriate surgical approach to mediastinitis following open-heart surgery and a wide range of wound-healing strategies have been established for the treatment of poststernotomy mediastinitis during the era of modern cardiac surgery. Conventional forms of treatment usually involve surgical revision with open dressings or closed irrigation, or reconstruction with vascularized soft tissue flaps such as omentum or pectoral muscle. Unfortunately, procedure-related morbidity is relatively frequent when using conventional treatments and the long-term clinical outcome has been unsatisfying. Vacuum-assisted closure is a novel treatment with an ingenious mechanism. This wound-healing technique is based on the application of local negative pressure to a wound. During the application of negative pressure to a sternal wound several advantageous features from conventional surgical treatment are combined. Recent publications have demonstrated encouraging clinical results, however, observations are still rather limited and the underlying mechanisms are largely unknown. This review provides an overview of the etiology and common risk factors for deep sternal wound infections and presents the historical development of conventional therapies. We also discuss the current experiences with VAC therapy in poststernotomy mediastinitis and summarize the current knowledge on the mechanisms by which VAC therapy promotes wound healing. Finally, we suggest a structured algorithm for using VAC therapy for treatment of poststernotomy mediastinitis in clinical practice.
1 Poststernotomy mediastinitis—the definition
Poststernotomy mediastinitis, also commonly called deep sternal wound infection, is one of the most feared complications in patients undergoing cardiac surgery. The definition of mediastinitis has been established by the Center for Disease Control and Prevention in the USA [1]. According to these guidelines, diagnosis of mediastinitis requires at least one of the following:
an organism isolated from culture of mediastinal tissue or fluid;
evidence of mediastinitis seen during operation;
one of the following conditions: chest pain, sternal instability, or fever (>38 °C), in combination with either purulent discharge from the mediastinum or an organism isolated from blood culture or culture of mediastinal drainage.
2 Microbial etiology
The microbial etiology of sternal wound infections varies and includes Gram-negative and Gram-positive bacteria (Table 1 ) as well as fungi. However, the most common causative pathogen involved in sternal wound infections is Staphylococcus epidermidis (CoNS) and Staphylococcus aureus, both from the normal flora of the skin [2–5]. Previously, the finding of CoNS in the wound could be dismissed as contamination and the pathogen was regarded as a relatively benign pathogen. However, S. epidermidis (CoNS) is now well known to be one of the most important agents of healthcare associated infections especially when foreign material is implanted, such as prosthetic heart valves, prosthetic joints, peritoneal dialysis catheters, intravascular catheters, and cerebral spinal fluids shunts. Another foreign body, steel wires, is used in almost all cardiac surgery procedures when closing the sternotomy and CoNS has emerged as the most important pathogen in poststernotomy mediastinitis, responsible for 43% to 64% of all cases in recent studies [2,3]. In a study from Shafir et al., a shift from Gram-negative bacteria to Gram-positive bacteria, especially CoNS, was observed in postoperative mediastinitis [6]. Following initial colonization, large amount of extracellular polysaccharide is synthesized, forming a protective biofilm around the colony. Therefore, treatment of infections caused by CoNS frequently necessitates removal of the infected foreign body. Furthermore, the antibiotic treatment requires susceptibility testing, because S. epidermidis strains are often resistant to multiple antibiotics. Previous studies have demonstrated that approximately 75% of the CoNS strains were methicillin resistant [2,3]. Coagulase-negative staphylococcal infections generally have a slow onset and are often associated with relatively few clinical signs of mediastinitis compared to mediastinitis caused by other bacteria [5]. However, no difference in mortality was observed between sternal infections caused by CoNS, when compared to S. aureus, or Gram-negative pathogens [2]. The other major pathogen in poststernotomy mediastinitis is S. aureus, which may have a more aggressive nature and demonstrate more classical signs of infection. This bacteria has been increasingly associated with colonization of the nasal passages of the patient [4]. The incidence of nasal colonization with S. aureus in the normal population is reported to range from 10% to 15% and such colonization increases the risk of poststernotomy mediastinitis [7]. Perioperative application of nasal mupirocin eradicates 95–100% of S. aureus up to 1 year postoperatively and demonstrates a 67% reduction of infection [8].
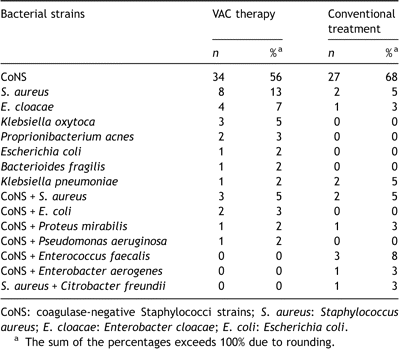
Culture-verified poststernotomy mediastinitis at Lund University Hospital, 1994–2003
3 Risk factors
The pathogenesis of poststernotomy mediastinitis is complex and multifactorial. In previous literature, several risk factors have been identified and in Table 2 a number of commonly observed risk factors are presented. However, even if a number of specific patient and procedure related risk factors have been delineated, inconsistency still remains regarding the contribution of individual factors to the development of sternal infections. Due to demographic changes in the Western countries the population undergoing cardiac surgery continuously grows older. At time of surgery a substantial part of this cohort also suffers from one or several co-morbidities, often related to their cardiovascular disease. Diagnoses such as obesity, diabetes mellitus, chronic obstructive pulmonary disorder, or heart failure are abundant. Previous literatures have demonstrated that the presence of one of these diagnoses, or risk factors, increase the risk for developing poststernotomy mediastinitis [9]. The mechanism by which obesity leads to this complication is not fully understood, however, perioperative antibiotics may be poorly distributed in adipose tissue, deep skin folds may cause the preoperative skin preparation to be inadequate, and it may also be difficult to diagnose mediastinitis in obese patients during the early phase of the infection [10]. Inadequate sterility may also be one of the mechanisms behind the finding of re-exploration for bleeding as a risk factor [5,11,12]. Diabetes mellitus is also a frequently reported risk factor for mediastinitis [9,13,14]. Elevated blood glucose levels may impair wound healing and the use of continuous, intravenous insulin has been shown to significantly reduce the incidence of deep sternal wound infection in diabetic patients [15]. Heart failure, a high NYHA Class, and a low ejection fraction (≪30%) of the left ventricle have also been demonstrated to be associated with mediastinitis [10,12,16]. A reduced ejection fraction is included as a variable in the mediastinitis score proposed by the Northern New England Cardiovascular Disease Study Group and presented in the ACC/AHA Guidelines for CABG surgery [17]. Furthermore, the use of bilateral internal mammary arteries has been reported as a risk factor for sternal infection. The mechanism is probably related to a devascularization of the wound margins after harvesting both mammary arteries, which may delay proper wound healing [12,13]. Extensive coronary atherosclerosis and re-do surgery has also been associated with an increased risk of mediastinitis [10,18]. Again, it is not clear what the true primary cause is, however, this risk factor probably reflects, at least to some extent, a general atherosclerotic condition, which may predispose these patients to poor wound healing in combination with longer procedure times. Prolonged duration of the surgical procedure should intuitively increase the risk of intraoperative contamination and length of surgery has been demonstrated in previous studies to be a risk factor for poststernotomy mediastinitis [10,13].
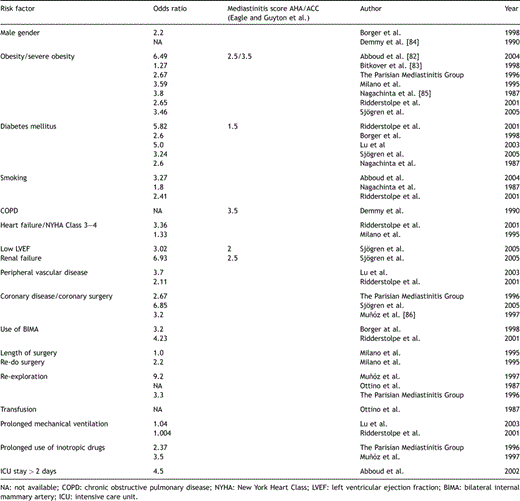
Independent risk factors for mediastinitis observed in previous studies
4 Short- and long-term survival following poststernotomy mediastinitis
With modern hospital hygiene standards and the use of prophylactic antibiotics the overall incidence of poststernotomy mediastinitis is usually reported to be low, usually between 1% and 3% [12,16,19,20]. However, even if this complication is relatively rare it is associated with significant mortality, reported to vary between 10% and 35% [5,9,12,13,21]. Deep sternal wound infection does not only lead to high mortality, but the morbidity in surviving patients is also significant [5]. Furthermore, poststernotomy mediastinitis is associated with a prolonged length of hospital stay [13], an increased cost of care [3,16] and significant impairment in long-term survival [10,13,19,22,23].
5 Conventional treatment in poststernotomy mediastinitis
During the development of modern cardiac surgery a number of wound-healing strategies have been established for the treatment of poststernotomy mediastinitis. Conventional forms of treatment usually involve surgical revision with open dressings or closed irrigation, or reconstruction with vascularized soft tissue flaps such as omentum or pectoral muscle. These wound-healing techniques may be used as a single-line therapy or in combination with other procedures. At the present time, there is little consensus regarding the appropriate surgical approach to mediastinitis following open-heart surgery.
Poststernotomy mediastinitis was initially treated with surgical revision, with or without multiple open dressing changes, followed by sternal re-wiring or secondary healing. However, a high mortality rate up to 45% has been reported following this strategy [24]. One major disadvantage of open dressings is thoracic instability, which requires mechanical ventilation. Prolonged immobilization may increase the risk of additional complications such as pneumonia, thrombosis and muscular weakening. Another devastating complication when leaving the sternum open is right ventricular laceration, which is associated with high mortality rates [25–27]. An important advance in mediastinitis treatment was made in 1963 with continuous irrigation in combination with drainage and a closed sternum [28]. Bryant et al. achieved further development with antibiotic irrigation [29]. Surgical revision with re-wiring or closed irrigation offers an expeditious procedure with the advantage of a closed wound and a stable sternum, but several studies have reported unsatisfactorily high rates of failure [25,30,31] and mortality [5]. The explanation to these failures may be an increased bacterial resistance and a relative devascularization of the left chest wall after harvesting the left mammary artery, which is nowadays a routine procedure. Therapeutic failure during poststernotomy mediastinitis treatment with recurrent infections is known to aggravate an already difficult situation, and results in very high early mortality [32]. Another alternative technique for healing wound infections, without continuous irrigation, is closed drainage with Redon catheters. This technique is based on surgical debridement in combination with multiple small catheters connected to bottle with a strong (−700 mmHg) negative pressure inside. The successful use of this therapy in poststernotomy mediastinitis was demonstrated in 1989 by Durandy et al. [33] and has been confirmed in more recent studies [34,35].
A commonly accepted wound-healing approach is primary, or delayed, wound closure with vascularized soft tissue flaps. The use of pectoral muscle flaps was initially described in 1980 [36]. Recent studies have reported varying results with pectoral muscle flaps in poststernotomy mediastinitis [37–39]. Other authors have advocated the technique employing omentum flaps first described by Lee et al. for closure of mediastinal defects [24,40,41], or reversed rectus abdominis muscle flaps [30,42]. Reconstruction with soft tissue flaps has a relatively low mortality rate according to some reports [37,41]. However, there are disadvantages, including additional surgical trauma and late flap-related morbidity such as pain, weakness and hernias [41,43,44]. Furthermore, there are several reports demonstrating poor long-term outcome with these techniques as mentioned previously.
6 Development of vacuum-assisted closure therapy
Vacuum-assisted closure (VAC) is a recent technical innovation in wound care with a growing number of applications. This wound-healing system was developed in the U.S. by Argenta and Morykwas in the mid 1990s [45]. During approximately the same time period a similar system using sub-atmospheric pressure in wound healing was evaluated by Fleischmann in Germany [46]. VAC therapy has been available in North America since 1995 and the system was introduced in Europe in 1997. This wound-healing technique is based on the application of local negative pressure to a wound. This is achieved by placing polyurethane foam with an open pore structure of 400–600 μm in the wound. One end of a non-collapsible tube is then connected to the foam and the other end is connected to a vacuum-source in a closed system via connected to a fluid container. The foam and the entire wound are covered with an adhesive drape thus ensuring an air-tight system. Finally, a predetermined, continuous or intermittent, negative pressure is applied to the wound. The foam dressing collapses on application of the negative pressure and transmits an even distribution of pressure across the wound [47].
7 Mechanisms of vacuum-assisted closure therapy
During the application of negative pressure to a sternal wound several advantageous features of conventional surgical treatment are combined. Vacuum-assisted closure allows open drainage that continuously removes exudate with simultaneous stabilization of the chest and isolation of the wound. By maintaining a moist environment, this therapy stimulates granulation-tissue formation [48] in combination with an increased blood flow in the adjacent tissue [49,50]. Furthermore, VAC therapy approximates the wound edges and provides a mass filling effect with a low degree of surgical trauma, without establishing a new wound (e.g. abdominal wound in omental flaps). Finally, due to sternal stabilization and wound isolation, patients can be mobilized early and receive physiotherapy in order to minimize further complications. Morykwas et al. also demonstrated a decrease in bacterial count during VAC therapy [48]. However, there have been conflicting results regarding the bacterial burden during VAC treatment [51,52]. Whether the arbitrary critical level of 1 × 105 CPU/g still is valid or not when using VAC in an infected wound is debatable [51].
The exact mechanism by which VAC exerts its wound-healing mechanism is not fully understood. It has been postulated that the increased perfusion results from an increased hydrostatic pressure gradient along an arteriole, thus directly drawing blood along the vessel [53]. An alternative explanation is the reduction of tissue edema by removing osmotically active molecules and mediators, thus preventing capillary compromise [48,54]. The tissue oedema in the wound margins creates a situation similar to a localized compartment syndrome [55]. A complimentary mechanism aiding the healing process would be the removal of inhibitory molecules. Bennet al. reported a decrease in inflammatory phase cytokines and a simultaneous increase in stimulating cytokines [56]. However, so far no quantitative studies have been reported supporting the theory of a reduction in interstitial fluid. Vacuum-assisted closure also approximates the wound margins, and therefore, exerts a mechanical force on the surrounding tissue. Tissue expansion is known to stimulate angiogenesis and increase the mitotic activity in skin [57,58]. It has also been hypothesized that mechanical stress (transduction) caused by VAC stimulates wound healing through ‘reversed’ tissue expansion [59,60]. Mechanical stimulation is known to stimulate several physiological mechanisms, such as ion transport, release of second messengers, alterations in gene expression, and increased protein synthesis. Shear stress has been demonstrated to increase cell division by up-regulation of the second messengers causing phosphorylation [61].
8 Vacuum-assisted closure therapy in poststernotomy mediastinitis
Several recent studies have reported the clinical benefits of VAC treatment in poststernotomy mediastinitis. In these studies, the VAC technique has been successful, either as a single-line therapy, or as a procedure for providing optimal conditions for second-line treatment with delayed flap closure [33,62–71]. Also successful data including high-risk patients and patients with infectious complications after assist device implantation or transplantation have been reported [72–75].
Our research group has previously presented a concept for VAC as a single-line therapy followed by sternal re-wiring without the use of additional tissue flaps [76]. We have used a structured approach with VAC, but without additional tissue flaps, for poststernotomy mediastinitis at our department in Lund since 1999 with promising results (Fig. 1 ). The survival data in Fig. 1 is based on 65 patients (mean age 67.9 ± 9.9) undergoing VAC therapy for poststernotomy mediastinitis between January 1999 and March 2004. The number of days from primary cardiac surgery procedure until mediastinitis diagnosis was 16.4 ± 10.6. The patients had VAC therapy (continuous suction) for 11.9 ± 9.0 days and the patients underwent a mean of 3.4 ± 2.2 procedures including initial debridement, VAC-changes, and final re-wire. All procedures were made in the operating theatre. All patients (100%) were re-wired without the use of tissue flaps and the total length of stay at our hospital was 24.6 ± 16.4 days. Our group has recently demonstrated an improved 90-days survival and a lower failure rate when comparing VAC therapy to conventional treatments [77]. We have also compared the long-term survival between patients undergoing CABG without mediastinitis to VAC-treated patients suffering from poststernotomy mediastinitis after CABG [78]. No difference in late survival was demonstrated, even after adjusting the Kaplan–Meier plot for differences in EuroSCORE. These results are contradictory to previously published reports regarding long-term survival after mediastinitis [13,25,26]. The reason for negative survival effects when using conventional treatments for poststernotomy mediastinitis is unclear. However, a severe systemic infection with repeated septic episodes due to ineffective treatments might cause irreversible effects in vulnerable organs such as the heart, kidneys, and bypass grafts [13]. We believe the successful long-term results found in our study may be due to the use of vacuum-assisted closure therapy as a wound-healing strategy. The effective combination of several wound-healing principles provided by VAC seems to result in minimal sequelae from the poststernotomy mediastinitis.
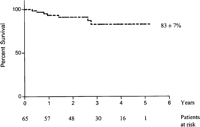
Overall survival after vacuum-assisted closure therapy for poststernotomy mediastinitis following cardiac surgery.
Catarino et al. performed an early, small retrospective study in order to compare VAC to continuous irrigation [33]. They reported a significantly greater number of treatment failures with continuous irrigation than with VAC. Domkowski et al. conducted an observational study using VAC therapy as a single-line treatment or as a bridge to tissue flap surgery, which showed very low (3.7%) early mortality [62]. However, they included both superficial and deep sternal wound infections. Fleck et al. reported lower rates of recurrent mediastinitis with VAC followed by delayed primary closure or pectoral muscle flaps compared to revision and primary closure [79]. Doss et al. reported good results when comparing VAC to conventional wound management, but they used VAC as a bridge to tissue flap treatment in 20% of their patients [65]. They also reported a shorter length of hospital stay and treatment duration following VAC therapy [65]. The shorter hospital stay with VAC treatment was verified in a recent study by Fuchs al., however, some of their patients were discharged with an open sternum relying on secondary wound healing without re-wiring [80]. Moidl et al. recently reported lower costs when using VAC therapy for poststernotomy mediastinitis [81]. In our retrospective comparison all patients underwent sternal re-wiring without the use of soft tissue flaps and no significant difference in length of stay or treatment duration was observed between VAC therapy and conventional treatment (Ref. [77]). One explanation may be that differences in health care systems affect the routines regarding discharge to the patients’ home or transfer of patients to the referring hospital. Furthermore, treatment duration varies considerably between different surgical techniques, and therefore, results between different studies should be interpreted with caution.
9 The Lund University Hospital mediastinitis algorithm
One important factor in successful outcome following poststernotomy mediastinitis is early referral to a surgical center with a structured approach and a well-known experience in wound-healing management. Delayed diagnosis and therapy will most likely lead a deteriorating patient resulting in increased morbidity and mortality. In our region all our referring hospitals discuss, and eventually, send patients with diagnosed, or suspected, mediastinitis back to our cardiothoracic center. We have adopted VAC therapy for poststernotomy mediastinitis in a structured fashion and here we describe the wound-healing concept used at Lund University Hospital (Fig. 2 ). While applying this algorithm all patients with mediastinitis have received vacuum-assisted closure therapy followed by successful delayed primary closure, including sternal re-wire, without the use of vascularized tissue flaps.
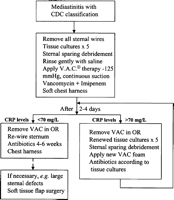
Algorithm for vacuum-assisted closure therapy in poststernotomy mediastinitis.
10 Conclusion and future perspectives
Poststernotomy mediastinitis is still one of the most feared complications after cardiac surgery and during the last 50 years several conventional treatments have been developed in order to manage these devastating infections. During the last decade a new treatment modality using topical negative pressure, or VAC, in the wound has evolved. Vacuum-assisted closure combines several advantageous features of conventional surgical treatments in addition to the unique features of topical negative pressure. During the last years VAC therapy has been adopted for use in poststernotomy mediastinitis and the use of this wound-healing therapy is currently spreading throughout the cardiothoracic community. Knowledge and experience regarding VAC in poststernotomy mediastinitis is constantly expanding and an increasing number of institutions report improved results when using this wound-healing strategy. However, several of these reports are relatively small studies and of an observational character including limited populations with short follow-up times. Therefore, although the use of VAC treatment in poststernotomy mediastinitis has presented promising clinical results, there is still need for further knowledge, e.g. large multi-center studies and randomized trials. Another way to ensure large enough amount of data is to initiate a European VAC Therapy Database Register. However, without a structured clinical approach at the individual center one may fail to secure all the clinical benefits of VAC therapy. Therefore, we present an algorithm for use in VAC-treated poststernotomy mediastinitis after cardiac surgery.




