-
PDF
- Split View
-
Views
-
Cite
Cite
Nilanjan Chaudhuri, Justin James, Adnan Sheikh, Antony D. Grayson, Brian M. Fabri, Intestinal ischaemia following cardiac surgery: a multivariate risk model, European Journal of Cardio-Thoracic Surgery, Volume 29, Issue 6, June 2006, Pages 971–977, https://doi.org/10.1016/j.ejcts.2006.03.014
Close - Share Icon Share
1 Introduction
Gastro-intestinal (GI) complications after cardiac surgery are uncommon (0.53–3.7%) but carry significant morbidity and high mortality (13.9–59%) [1–11]. The variability in mortality is due to the large variety of GI complications and their associated management. However, intestinal ischaemia with a reported mortality of 64–100% [5,12–14] remains the most lethal GI complication following cardiac surgery.
Improvements in surgical techniques such as off-pump surgery do not seem to have made any significant impact on its incidence or prognosis [15]. One of the reasons may be that despite numerous retrospective reports of GI complications in general and of intestinal ischaemia in particular following cardiac surgery few have analysed the predictive factors systematically as pre-operative, intra-operative and post-operative [1–14]. Most authors agree that the difficulty in making the diagnosis in critically ill ventilated and sedated patients following cardiac surgery contributes heavily to the catastrophic end result. Early diagnosis and prompt surgical intervention can improve outcome in these patients [12].
We aimed to develop a multivariate risk model to identify those patients at highest risk of developing intestinal ischaemia.
2 Materials and methods
2.1 Patient population and data
This study was conducted on a population of 10,976 consecutive cardiac surgery patients who underwent operation between April 1997 and March 2004. All data were collected prospectively during the patient admission and entered onto a cardiac surgery database as part of routine clinical practice. Methods of data collection and definitions are available from http://www.nwheartaudit.nhs.uk/Progressreport1997-2001.pdf.
Pre-operative data were collected on the following variables: age, sex, body mass index, urgency of operation, prior cardiac surgery, angina class, history of myocardial infarction, smoking, diabetes, hypercholesterolaemia, hypertension, peripheral vascular disease, cerebrovascular disease, respiratory disease, renal dysfunction, previous gastric ulcer, previous GI surgery, the extent of coronary disease and left ventricular ejection fraction.
Data on the type of procedure, use of cardiopulmonary bypass (CPB), duration of CPB and aortic cross-clamp (AXC) were also collected. Post-operative data collected included development of intestinal ischaemia, in-hospital mortality, re-exploration for bleeding, atrial arrhythmia, renal failure, highest creatine kinase-MB (CK-MB) release on post-operative day 1, blood loss in the intensive care unit (ICU), duration of mechanical ventilation, and use of inotropes and intra-aortic balloon pumps (IABP). Blood transfusion data was obtained from the local blood transfusion service, which is provided routinely on a monthly basis. These data consisted of date of request for platelets and number of units used, along with the number of red blood cell (RBC) and fresh frozen plasma (FFP) units used. All intra-operative and post-operative variables were censored at the time of onset of intestinal ischaemia.
A diagnosis of intestinal ischaemia was made either clinically based on laparotomy findings or at post-mortem examination. All patients showing signs of ischaemic bowel in the form of discolouration, mucosal necrosis or gangrene was included in the study. In-hospital mortality was defined as death within the same hospital admission regardless of cause. All patients transferred from the base hospital to another hospital were followed up to confirm their status at discharge. Re-exploration for bleeding was defined as bleeding that required surgical re-operation after initial departure from the operating theatre. Post-operative atrial arrhythmia was defined as the occurrence of new atrial arrhythmia in the absence of pre-operative persistent or paroxysmal atrial arrhythmias. Renal failure was defined as patients with a post-operative creatinine level greater than 200 μmol/l or patients requiring dialysis.
2.2 Anaesthesia, cardiopulmonary bypass and surgery
On-pump or off-pump coronary artery bypass grafting (CABG), valve surgery, thoracic aortic or thoraco-abdominal aortic surgery, surgery for adult congenital heart defects and tumours of the heart or a combination of any of the above procedures constituted the study population. CPB was maintained with nonpulsatile blood flow between 2.0 and 2.2 l/(min m2) body surface area with a median perfusion pressure of between 50 and 70 mmHg and systemic cooling to 28–32 °C. The ascending aorta or occasionally, the femoral artery was used for arterial cannulation and right atrial or bicaval cannulation for venous return. Cold, intermittent, antegrade, and retrograde blood or crystalloid cardioplegia with or without topical cooling was the method of myocardial protection.
2.3 Statistical analysis
Due to non-normality (tested with Shapiro–Wilk), continuous data are shown as median values with 25th and 75th percentiles and comparisons made with Wilcoxon rank sum tests. Categorical variables are shown as a percentage and comparisons were made with chi-square tests as appropriate. A multivariate logistic regression analysis was undertaken, using the forward stepwise technique, to identify independent risk factors for intestinal ischaemia. Candidate variables were entered into the model with a p-value less than 0.1, while the p-value for backward elimination was set at 0.05. Variables offered to the model were checked for potential interactions. If continuous variables were found to be significant, they were then assessed as dichotomous variables in units of 5. Continuous variables were either included as dichotomous variables or in their original form in the final model depending on the strength of association. The area under the receiver operating characteristic (ROC) curve and the Hosmer–Lemeshow goodness-of-fit statistic were calculated to assess the performance and calibration of the model, respectively. In all cases a p-value <0.05 was considered significant. All statistical analysis was performed with SAS for Windows Version 8.2.
3 Results
During the study period a total of 50 patients developed intestinal ischaemia amongst 10,976 consecutive cardiac surgery patients (incidence 0.5%). Fifteen of these underwent laparotomy and the remaining patients were diagnosed to have intestinal ischaemia on a post-mortem examination. Of the 15 laparotomies, 5 (33%) were considered to be therapeutic. The median time to laparotomy was 8 days, with the longest being 42 days.
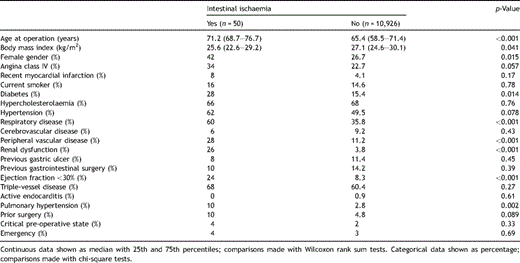
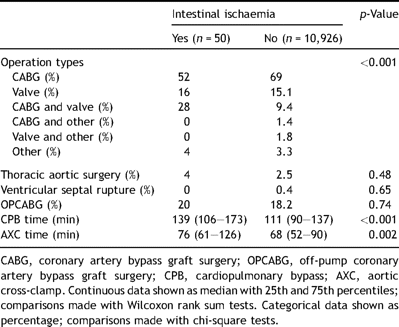
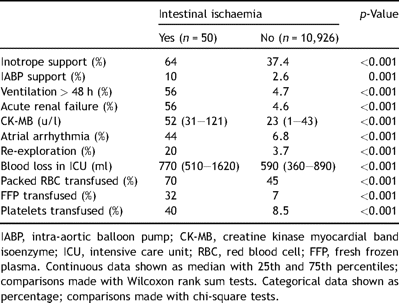
Tables 1–323 show the pre-operative, operative, and post-operative characteristics of our patient population based on whether they developed intestinal ischaemia, respectively.
The independent risk factors for intestinal ischaemia, along with coefficients, standard errors, odds ratios, confidence limits, and p-values, are shown in Table 4. At the bottom of Table 4 is the logistic regression equation for calculation of predicted risk of intestinal ischaemia. The area under the ROC curve for the multivariate prediction model was 0.93 (Fig. 1). The predicted risks of individual patients were rank-ordered and divided into deciles. Within each group of estimated risk, the number of intestinal ischaemia predicted was compared with the number of observed intestinal ischaemia. The Hosmer–Lemeshow goodness-of-fit statistic across deciles of risk was not statistically significant (Fig. 2, p = 0.32), indicating little departure from a perfect fit.

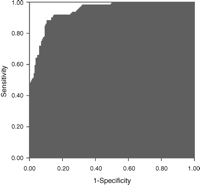
Receiver operating characteristic curve for pre-operative multivariate model (ROC = 0.93).
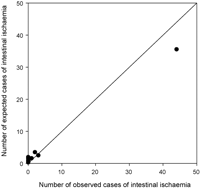
Observed versus expected intestinal ischaemia (Hosmer–Lemeshow = 0.32).
Fig. 3 shows the observed incidence of intestinal ischaemia based on low-risk (less than 95th percentile of predicted risk), medium-risk (95th to 98.9th percentile of predicted risk), and high-risk (greater or equal to the 99th percentile of predicted risk) bandings.
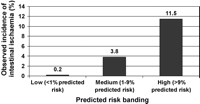
Observed incidence of intestinal ischaemia based on low-, medium-, and high-risk bandings.
The overall in-hospital mortality rate for our institution during the study time period was 4%. In-hospital mortality for the patients who developed intestinal ischaemia was 94% (47/50) compared to 3.6% (390/10,926) for the other patients (p < 0.001). The mortality rate was lower in the 15 patients who received laparotomy at 80% (12/15), however, this was still significantly higher compared to patients with no intestinal ischaemia (p < 0.001).
4 Discussion
Acute intestinal ischaemia is an uncommon but catastrophic complication after cardiac surgery. Previous studies have demonstrated an incidence of 0.16–2.0% [1,2,12]. In our population of 10,976 consecutive cardiac surgery patients we found that 0.5% had intestinal ischaemic complications. Recent reports [2,12] as well as this study show a lower incidence (less than 1%) to that reported a decade ago [1]. In-hospital mortality in our group of patients was 94%; or 80% if the patients underwent laparotomy. Previous studies have reported mortality rates between 64 and 100% [5,12–14].
Our primary objective in this study was to develop a multivariate risk model, which might enable us to predict those patients who had a high risk of developing post-operative intestinal ischaemia. On multivariate analysis we identified five predictive factors for GI ischaemia post-operatively.
Advanced age, especially patients over 80 years, has been reported as an independent predictor of intestinal ischaemia following cardiac surgery [2,4]. The exact reason for this association is not known but diminished physiologic reserve and occult co-morbid conditions, such as visceral atherosclerosis may contribute.
Impaired left ventricular function has been associated with GI ischaemia in many reports [3,8,10]. It is generally agreed that post-operative intestinal ischaemia in cardiac surgical patients is chiefly a result of mesenteric hypo-perfusion leading to non-occlusive mesenteric ischaemia (NOMI) [3,5,7,13]. A low cardiac output state predisposes to this pathology [4].
The pathogenesis of GI ischaemia after cardiac surgery is multifactorial, but it is generally agreed that reduced systemic blood flow leading to tissue hypoxia and energy deficit is the major responsible factor. There are several reasons why the gut is more susceptible to ischaemia. Oxygen shunting and tissue hypoxia can occur in intestinal villi even when normal perfusion is present [16]. The GI tract is unable to compensate for hypotension by autoregulation and furthermore, vasoconstriction may persist even when haemodynamic stability is re-established [4]. Increased inotropic requirement and the need for intra-aortic balloon pump counterpulsation reflect the need to support a low output state. This increased support in patients who develop intestinal ischaemia has been documented in other reports as well [4,9,11,12].
The combination of post-operative inotropic support and acute renal dysfunction was a significant predictor of mesenteric ischaemia in our model. Acute renal failure may be a feature of the generalised organ hypoperfusion in the post-operative period rather than a direct contributor to the complication. However, acute renal failure has also been shown to contribute to GI injury in animal experiments [17] and has been shown to be an independent predictor of GI ischaemia on its own, in previous reports [4,13].
There have been conflicting reports of the direct association between intestinal ischaemia and prolonged CPB. Some groups [8,10] have found a strong relationship with odds ratio of 1.3–1.7, while others [3,4,11] did not find any significant association on multivariate analysis. In this study both the length of CPB and aortic cross-clamp times were significantly associated with intestinal ischaemia on univariate analysis. However, they did not appear significant in the multivariate risk model. It would therefore appear that the mere length of cardiopulmonary bypass might not be as important as the adequacy of overall organ perfusion. It stands to reason however that shorter times of non-physiological perfusion are less likely to result in inadequate organ perfusion.
To our knowledge, pre-operative respiratory disease as a risk factor for post-operative GI complications has been reported by only one study [6]. Significant respiratory co-morbidity increases post-operative morbidity and mortality in cardiac surgical patients [18,19]. The univariate association between respiratory disease and intestinal ischaemia disappeared on multivariate analysis though due to the strong association between the patients who developed intestinal ischaemia and patients who required more prolonged mechanical ventilation. Spotnitz et al. [11] and D’Ancona et al. [4] have both reported prolonged mechanical ventilation as independent predictors of GI complications with OR of 6.6 and 5.5, respectively, while in another study [12] this factor was found to be significantly associated with intestinal ischaemia on univariate analysis. This may reflect sicker patients requiring increased support and while being significantly associated with post-operative intestinal ischaemia are not necessarily causative factors.
Similar to this study, post-operative atrial fibrillation has been shown recently by Andersson et al. [2] to be an independent predictor of GI complications after cardiac operations.
Post-operative loss of blood in excess of 700 ml was an independent predictor of intestinal ischaemia in our model. There was also a significantly higher usage of blood, fresh frozen plasma and platelets in the group of patients that developed intestinal ischaemia when compared to the cohort that did not on univariate analysis. Post-operative transfusion of blood and blood products has been shown to have a significant association with GI ischaemia in other studies [2,12]. In our study these factors do not stand out as independent predictors because of their subordinate relationship with increased post-operative blood loss.
Patients who develop intestinal ischaemia have significantly higher in-hospital (94% vs 3.6% (p < 0.001)) in our study groups. Unfortunately, patients who present with signs and symptoms of intestinal ischaemia [14] are often beyond any salvage strategy. Survival however depends upon early recognition [6] and even more importantly upon early laparotomy [12]. Abdominal pain is generally accepted as the earliest and most sensitive indicator of a significant GI event [6]. However, this may be masked in a lot of patients who are often ventilated or sedated. In such situations there are no non-invasive tests to reliably detect intestinal ischaemia, especially in the setting of acute NOMI. Mesenteric angiography is the only test, which can be diagnostic [20]. Unsurprisingly, it is not routinely used because of its invasive nature and the logistics involved in the context of what is commonly an unstable patient. A high index of suspicion and a low threshold for laparotomy may be what is required to improve the outcome in these patients.
Hasan et al. [14] have recently described three modes of presentation of intestinal ischaemia after cardiac surgery. The first one is a non-specific presentation in association with a low-output syndrome and multi-organ failure, where intestinal ischaemia is a terminal event and the end result is usually fatal. These cases are difficult to treat and carry a poor prognosis. The second type of presentation is with acute abdominal pain and the authors’ recommend this to be treated with prompt laparotomy within 6 h of onset of symptoms for best chances of a ‘cure’. The final presentation is with abdominal distension and pseudo-obstruction. This is felt to be representative of a poor cardiac output and splanchnic ischaemia. The authors’ recommend aggressive treatment of the hypoperfused state. Mesenteric angiography has been suggested as a means to detect NOMI, if symptoms do not settle in 3 days [20]. If NOMI is detected, local papaverine infusions have shown good results [20]. The role of dopexamine and dopamine in causing splanchnic vasodilatation in intestinal ischaemia remains unproven [21].
There are several limitations to our study. Firstly, being a retrospective database study, by its nature, it is only capable of showing association between variables and outcomes, and is unable to demonstrate cause and effect. Furthermore, the retrospective nature of the study cannot account for uncollected or unknown variables affecting the outcome. It is possible that ours being a specialist cardiothoracic hospital, as opposed to a general hospital with a specialist tertiary care unit [12] may have had an influence on the lower laparotomy rate 15/50 (30%) and high mortality. The multivariate model itself may lack statistical power with only 50 outcomes and 5 risk factors identified.
Most of the post-operative factors occur as a cluster usually in a haemodynamically unstable patient. So the extent of contribution of each of these factors as either an aetiological factor or merely as a surrogate marker of general haemodynamic instability is uncertain. Only a prospective study with sufficient statistical power looking chronologically as the events unfold in these patients can resolve these issues.
In conclusion, the incidence of intestinal ischaemia following cardiac surgery is low. However, the condition remains serious and carries a far worse prognosis when compared to a general cohort of patients undergoing cardiac surgery. We have identified several predictors which may be useful in identifying patients at high-risk of developing intestinal ischaemia. Furthermore, we have quantified this risk with a prediction equation (Table 4), which may prove useful in assessing the probability of developing this severe complication. Serious pre-emptive consideration to the peri-operative management of patients with a high risk (Fig. 3) of developing intestinal ischaemia is required. A high index of suspicion in all situations where the post-operative course deviates from the norm and a low threshold for performing an early laparotomy may have a favourable impact on this dreaded complication. A prospective study would be required to validate this model and check if such a strategy has a beneficial effect on outcome. However, given the low overall incidence of this ominous complication, this would require a multi-centre design for any useful information to be gained within a reasonable length of time.
We would like to acknowledge the co-operation given to us by all the Consultant Cardiac Surgeons at The Cardiothoracic Centre-Liverpool: Mr J.A.C. Chalmers, Mr W.C. Dihmis, Mr B.M. Fabri, Miss E.M. Griffiths, Mr N.K. Mediratta, Mr A.Y. Oo, Mr D.M. Pullan, Mr A. Rashid and Mr W.I. Weir. We would also like to thank Janet Deane and Andrew Ward, who maintain the quality and ensures completeness of data collected in our Cardiac Surgery Registry.
Appendix A
Conference discussion
Dr D. Wong (Halifax, Canada) Do you have any information regarding the aetiology of the ischaemic gut? Was it embolic or was it low flow? Do you have any information about that and whether that affects your risk factors?
Dr Chaudhuri: This is quite well established in the published literature, and even in our study. We had 15 laparotomies and we suspected intestinal ischaemia, but there wasn’t a post-operative diagnosis. But it is quite well established that following cardiac surgery, it's mainly a syndrome of nonocclusive mesenteric ischaemia resulting from a low-output syndrome. It's not embolic in most of them. In our case it was exactly the same. Over 90% were NOMI, nonocclusive mesenteric ischaemia, resulting from a low-output syndrome post-operatively.
Dr F. Eckstein (Berne, Switzerland) Maybe I missed it, but the inotropic support, was there something that influenced the intestinal ischaemia in your patients?
Dr Chaudhuri: I presume you are referring to the vasopressors.
Dr Eckstein: Yes.
Dr Chaudhuri: Well, we tried to look at this in detail. We only had 50 patients, and by the time these patients were suspected of developing intestinal ischaemia, they were on a whole range of inotropes, including vasopressors. But looking at the literature and also looking at our series, I think it would appear that it's more the low perfusion state rather than the actual going up on vasopressors which is the problem. So the problem was already there. You just accentuated it.
Dr Eckstein: That leads me to the next question. What is your therapy in these patients?
Dr Chaudhuri: What we suggest is that if you use this model, you have already identified pre-operatively the patients who are going to be at highest risk. You might be able to modify your operative strategy. Say, for instance, you might try and keep your operative time low. You might do it yourself rather than let the resident do the operation. You know, it's a high-risk patient. Modify the other operative strategy, that's one. The second thing is, when you start seeing patients in whom their post-operative course starts to vary, like you saw in these two groups of patients, inotrope support is going up, something is wrong, because often these patients are ventilated and intubated and they don’t tell you, ‘I have tummy pain,’ and so you have to suspect, and if you have a strong suspicion, there is no harm in doing a negative laparotomy. You can see from our study, we only had 15 laparotomies, which means, although we suspected the occurrence of intestinal ischaemia in our group of patients, it was too late. They had multiorgan failure, a very poor prognosis. It was too late to do anything. The suspicion is the key really.
Dr Eckstein: Have you performed any angiography and maybe intraluminal papaverine infusion?
Dr Chaudhuri: No. Again, you are quite right. There is a recent paper from Oxford that describes three syndromes, and one is them is what you mentioned, you know, the patient is describing bloating symptoms and other things. In those instances you can actually perform mesenteric angiography, although it is invasive, and if you prove NOMI, then you give papaverine. But these patients, if you remember, are already on a lot of inotropic support. It's quite unstable to get them to the mesenteric angiography suite.
Dr D. Lindblom (Stockholm, Sweden) Most of the risk factors you identified were those that are usually seen when you are doing risk factor analysis. The sicker the patient, the more complications you can expect. So the prediction is not very easy. I saw that you had a double frequency of off-pump surgery in the patients with intestinal ischaemia. Do you think that is a consequence of the patients being worse, or do you think that the perfusion or the lack of perfusion from the heart-lung machine has anything to do with this?
Dr Chaudhuri: Let me go to this slide. There was no double difference. There was no difference. That was propensity matching. We tried to match them as closely as possible. So I know there was 20 and 10, but there was no significant statistical difference in the group. So we are actually trying to match there rather than show the difference. But in the published literature, actually there are two reports, one from our institution and one from the Southampton group, comparing off-pump and on-pump surgery and, again, in both studies they have not found any difference between off-pump and on-pump surgery for the incidence of intestinal ischaemia.
Dr Lindblom: What is your frequency of off-pump coronary surgery?
Dr Chaudhuri: I can’t really comment on that, but I think it's about 30%.




