-
PDF
- Split View
-
Views
-
Cite
Cite
Manoj Kuduvalli, Nick Newall, Anthony Stott, Antony D. Grayson, Brian M. Fabri, Impact of avoiding cardiopulmonary bypass for coronary surgery on perioperative cardiac enzyme release and survival, European Journal of Cardio-Thoracic Surgery, Volume 29, Issue 5, May 2006, Pages 729–735, https://doi.org/10.1016/j.ejcts.2006.01.041
Close - Share Icon Share
Abstract
Objective: This study examines the association between avoiding the use of cardiopulmonary bypass (CPB) for coronary surgery and postoperative cardiac enzyme (CE) release, and its subsequent impact on survival. Methods: Between January 1999 and September 2002, 3734 consecutive patients underwent either off-pump or on-pump coronary surgery. Patient characteristics and postoperative cardiac enzyme release were collected prospectively. Logistic regression was used to assess the effect of off-pump coronary surgery on cardiac enzyme release. All analyses were adjusted for preoperative characteristics and number of grafts. All patients were followed up at 1 year to assess survival. Results: Nine hundred and sixty (25.7%) patients had off-pump coronary surgery. Seven hundred and twenty-six (19.4%) patients had cardiac enzyme release three to six times the upper limit of the reference range, while 266 (7.1%) patients had cardiac enzyme release more than six times the upper limit of the reference range. After adjusting for patient characteristics, off-pump surgery was associated with less release (cardiac enzyme release three to six times, adjusted odds ratio 0.43, p ≪ 0.001; cardiac enzyme release more than six times, adjusted odds ratio 0.59, p = 0.005). Risk adjusted survival at 1 year was 97.5% for the on-pump group and 97.0% for the off-pump group (p = 0.33). Conclusions: Avoiding cardiopulmonary bypass significantly reduces early cardiac enzyme release following coronary artery bypass grafting (CABG). However, it does not result in improved survival compared to coronary surgery using cardiopulmonary bypass. This absence of survival benefit may be due to higher mortality rates experienced by the fewer patients with high (≫6 times the upper limit of range) cardiac enzyme release following coronary artery bypass surgery without cardiopulmonary bypass.
1 Introduction
The release of cardiac enzymes (CE) in the form of creatine kinase (CK) and especially creatine kinase-MB (CK-MB) fraction has been shown to correlate well with the extent of myocardial injury [1]. The usefulness of CK-MB as a reliable marker of perioperative myocardial ischaemia and injury after coronary artery bypass grafting (CABG) has also been demonstrated [2]. Postoperative serum CK-MB activity has also been demonstrated to be an accurate method for confirming or excluding perioperative myocardial injury after off-pump CABG (OPCAB) [3]. There is some evidence in the literature that increased perioperative serum cardiac enzyme levels after CABG are associated with a significant increase in both repeat myocardial infarction and death beyond the immediate perioperative period [4]. Similar data exist for percutaneous coronary interventions [5,6].
A number of randomised control trials have shown lower perioperative CK-MB [7,8], troponin I [9] and troponin T [10] release following OPCAB compared to patients undergoing conventional CABG with the use of cardiopulmonary bypass (on-pump coronary artery bypass grafting, ONCAB). However, these studies were small and highly selected, and may not be representative of a routine coronary practice.
We set out to see whether OPCAB is associated with lower postoperative CK-MB release in routine clinical practice and sought to see if this is associated with a difference in 1-year survival.
2 Methods
2.1 Patient population and data
We conducted a large-scale cohort study using prospectively collected data on 3734 consecutive patients undergoing isolated CABG surgery between 1st January 1999 and 30th September 2002 at the Cardiothoracic Centre, Liverpool. All operations were performed through a median sternotomy. Details of the different surgical techniques used in these patients have been published previously [11]. OPCAB was performed consecutively by three of the seven surgeons at our institution. Any conversions to cardiopulmonary bypass (CPB) were classified as OPCAB cases on the basis of ‘intention to treat’ analysis (11 cases were converted).
Preoperative data was collected prospectively during patient admission as part of routine clinical practice. Methods of data collection and definitions have been previously published [12] and are also available at http://www.nwheartaudit.nhs.uk. Data were collected on the following variables: age, sex, body mass index, urgency of operation, hypertension, hypercholesterolemia, diabetes, peripheral vascular disease, respiratory disease, renal dysfunction, prior cardiac surgery, the extent of coronary disease and left ventricular ejection fraction. The number of coronary vessels grafted during the procedure was also documented.
Cardiac enzyme release (total CK and CK-MB fraction) within the first 24 h postoperatively was abstracted from a routinely recorded electronic clinical biochemistry archive, blind to survival and clinical data. The cardiac enzymes were measured on the morning after the operation, approximately 12–18 h after the patient's arrival in the Intensive Care Unit. The assays used were CK-MB measured by the immuno-inhibition method performed on the Roche 917 Analyser (reference range 5–24 U/l at 37 °C) and total CK (Roche, reference range ≪190 U/l in men, ≪167 U/l in women at 37 °C). Where both total CK and CK-MB were recorded, the value of CK-MB isoenzyme was selected in preference to the value of total CK.
2.2 Patient follow-up
Patient records were linked to the National Strategic Tracing Service (NSTS), which records all deaths in the United Kingdom. To establish vital status at 1 year after operation, patients were matched to the NSTS based on patient name, National Health Service number, date of birth, gender and postcode.
2.3 Statistical methods
Continuous variables not normally distributed are shown as median with 25th and 75th percentiles. Categorical data are shown as percentages. Univariate comparisons were made with Wilcoxon rank sum tests and chi-square tests as appropriate. Logistic regression was used to assess the relationship between OPCAB and postoperative cardiac enzyme release [13]. Deaths occurring over time for OPCAB and ONCAB patients were described using Kaplan–Meier survival curves and Cox proportional hazards regression to adjust for case-mix differences [14,15]. The logistic regression and Cox proportional hazards analyses were risk adjusted for the following variables: age, sex, body mass index, previous cardiac surgery, left ventricular ejection fraction, left main stem stenosis, number of major coronary arteries with stenosis ≫70%, priority of surgery, hypertension, hypercholesterolemia, peripheral vascular disease, diabetes, renal dysfunction, respiratory disease, preoperative white cell count and number of grafts [16]. These analyses were also repeated on elective cases only to account for any potential differences in preoperative CE release. The elective patients’ results were entirely consistent with our main findings and are therefore not shown.
As the surgical technique (OPCAB or ONCAB) was not randomly assigned in this patient population, potential confounding and selection biases were accounted for by developing a propensity score for OPCAB group membership [17]. The propensity for OPCAB group membership was determined without regard to outcome, using multivariable logistic regression analysis. A full nonparsimonious model was developed that included all variables listed in Table 1. The goal is to balance patient characteristics by incorporating everything recorded that may relate to either systematic bias or simply bad luck. This model yielded a C statistic of 0.78, indicating a good ability to differentiate between OPCAB and ONCAB patients. We then used a macro (available at: http://www2.sas.com/proceedings/sugi29/165-29.pdf) to perform one to many matching. This process matched two ONCAB patients for every one OPCAB patient, firstly using an identical 8-digit propensity score. If this could not be done, we then proceeded to a 7-, 6-, 5-, 4-, 3-, 2-, or 1-digit match.
In all cases a p-value ≪0.05 was considered significant. All statistical analysis was performed retrospectively using SAS for Windows version 8.2.
3 Results
3.1 Patient characteristics
During the study period, 960 (25.7%) patients underwent OPCAB, compared to 2774 (74.3%) patients who underwent ONCAB. Table 1 lists the patient characteristics based on the procedure performed. OPCAB procedures were more likely to be performed in younger patients, with less extensive coronary disease. However, OPCAB patients were more likely to be obese, more likely to have undergone prior cardiac surgery, and had higher preoperative white cell counts.
3.2 Crude and adjusted cardiac enzyme release
Seven hundred and twenty-six (19.4%) patients had CE release between three and six times the upper limit of the reference range (ULR) (72–144 U/l), while 266 (7.1%) patients had CE release more than six times the ULR (≫144 U/l). The percentages of patients with CE release between three and six times and more than six times ULR, based on the procedure performed, are shown in Table 2. OPCAB compared to ONCAB was significantly associated with less CE release (CE release between three and six times ULR, crude odds ratio (OR) 0.42, p ≪ 0.001; CE release more than six times ULR, crude OR 0.49, p ≪ 0.001). Of the 11 conversions contained within the OPCAB group, three patients had a CE release between three and six times ULR, and two patients had a CE release greater than six times ULR.
Table 2 also shows that after adjusting for patient characteristics, OPCAB compared to ONCAB was still associated with less CE release (CE release three to six times ULR, adjusted OR 0.43, p ≪ 0.001; CE release more than six times ULR, adjusted OR 0.59, p = 0.005).
When restricting the analysis to only those patients with CK-MB values measured (95% of patients in the study), OPCAB patients still had a significantly lower postoperative CE release compared to ONCAB patients. The median CK-MB level in OPCAB patients was 20 U/I (25th and 75th percentiles: 13–37) compared to 39 U/I (25th and 75th percentiles: 27–63) for ONCAB (p ≪ 0.001).
3.3 Crude and adjusted 1-year survival
One hundred and forty-nine (4%) deaths occurred within 1 year of follow-up (all follow-up was complete at 1 year). The crude hazard ratio (HR) of mortality at 1 year for OPCAB patients was 1.09 (95% confidence intervals (CI) 0.76–1.57; p = 0.63). Freedom from death in the ONCAB patients at 30 days and 1 year was 98.1% and 96.1%, respectively, compared with 98.5% and 95.7% for the OPCAB patients (Fig. 1a).
After adjustment for baseline differences in patient and disease characteristics, the adjusted HR of mortality at 1 year for OPCAB patients was 1.22 (95% CI 0.82–1.82; p = 0.33). The adjusted Kaplan–Meier survival curves are shown in Fig. 1b. Freedom from death in the ONCAB patients at 30 days and 1 year was 98.9% and 97.5%, respectively, compared with 98.7% and 97.0% for the OPCAB patients.
Fig. 2 shows survival curves at 1 year in patients with CE release three to six times ULR and CE release more than six times ULR stratified by whether the procedure was performed with or without cardiopulmonary bypass. No significant survival difference was evident between OPCAB and ONCAB patients with CE release three to six times ULR (p = 0.58). However, OPCAB patients did have a significantly lower survival both at 30 days and 1 year compared to ONCAB patients when CE release was more than six times ULR (p ≪ 0.001).
3.4 Propensity-matched analysis
After performing a one to two propensity-matched analyses, we successfully matched 600 OPCAB patients to 1200 ONCAB patients. Of the 600 OPCAB patients matched, 3 (0.5%) were 5-digit matches, 71 (11.8%) were 4-digit matches, 304 (50.7%) were 3-digit matches, 186 (31%) were 2-digit matches, and 36 (6%) were a 1-digit match. Table 3 shows that the patient characteristics of both groups were well matched, including extent of disease and number of grafts used. Table 4 shows the comparison between various territories of myocardium revascularised in the propensity-matched patients.
After accounting for potential selection bias, propensity-matched OPCAB patients still had significantly less incidence of CE release between three and six times ULR (6.3% vs 14.5%; p ≪ 0.001) and greater than six times ULR (4.8% vs 7.9%; p = 0.015) compared to propensity-matched ONCAB patients. With respect to the impact this has on survival, Fig. 3 shows that the freedom from death in OPCAB patients with CE release ≫6 times ULR was lower both at 30 days and 1 year compared to similar ONCAB patients, although this just failed to reach statistical significance (p = 0.09).
3.5 Unmatched OPCAB patients
There were important differences in patient characteristics in the matched and unmatched OPCAB patients (Table 5). Unmatched off-pump patients had more diabetes, renal dysfunction, respiratory disease, ejection fraction less than 30% and more prior cardiac surgery. Unmatched off-pump patients also had significantly less coronary disease and fewer bypass grafts.
4 Discussion
The present study shows that, in routine clinical practice, patients undergoing OPCAB have markedly reduced postoperative CE release compared to patients undergoing ONCAB, suggesting significantly lower levels of perioperative myocardial injury. The levels of CE release observed in this study are consistent with those observed following minimally invasive CABG [18], as well as in randomised controlled studies comparing OPCAB with ONCAB [7,8] suggesting that the observed differences in CE release are more likely to be causally associated with the use of cardiopulmonary bypass. Perioperative CK-MB release has previously been shown to be a reliable marker for myocardial ischaemia and injury in patients undergoing CABG, both with [2] and without [3] the use of CPB.
The question about whether these results would have any significant impact on the outcome for the patient is open to debate. There is growing evidence in the literature that even small elevations of serum CE in the periprocedural period that were hitherto considered insignificant could actually be markers for adverse outcomes in the short and medium term. Such evidence has been demonstrated after both surgical [4] and percutaneous [5,6] coronary interventions. Some of these studies, especially the ones dealing with percutaneous coronary intervention have included a large number of patients, who have been followed up for mean periods ranging from 12 months to 38 months [5,6]. In one of these studies, death within 4 months was independently correlated with the degree of CK-MB elevation [6]. Previous work from our own institution has demonstrated that both intermediate (three to six times ULR) and high (more than six times ULR) perioperative cardiac enzyme release are independently associated with increased 1-year mortality following isolated CABG surgery [19]. Another study demonstrated that an increased postoperative peak CK-MB level indicates increased risk of severe postoperative left ventricular dysfunction and mortality within 30 days of ONCAB [20]. Hence, based on this evidence, a group of patients who have a lower perioperative serum CK-MB rise should have a better survival compared to a group of patients having a higher perioperative CK-MB rise. However, this was not apparent in our study. There was no significant difference between risk-adjusted survival at 30 days and 1 year between the groups undergoing OPCAB or ONCAB. One possible explanation for the increased CE release in patients undergoing conventional CABG is to partly attribute it to trauma caused to the right atrium by cannulation, rather than assuming that the entire rise in CE is due to generalised myocardial injury. It has been shown in the past by Lee and colleagues that the human atrium is a rich source of CK, with the proportion of CK-MB similar to that present in the ventricle (20%) [21]. Another possible explanation could be that the elevation in CE in patients undergoing ONCAB is more due to reversible myocardial ischaemia causing a functional dysintegrity of the cell membranes resulting in the release of cytosolic enzymes without subsequent cellular necrosis or any relevant decrease in function [22,23]. The reversible nature of this injury may not have any impact on the clinical outcome.
Czerny and colleagues [7], in a small, randomised controlled study, similarly did not find any significant difference in the in-hospital clinical outcomes. They suggested that it seems likely that differences in clinical outcome—with or without CPB—may only be seen in high-risk patients undergoing CABG, e.g. the very elderly, in whom in-hospital mortality rates following conventional CABG were higher than average. However, our study population included a significant number of high-risk patients, and there was no difference in survival at 30 days or 1 year even after appropriate risk-adjustment was performed on the data.
Stratifying the study patients into four groups based on surgical technique and the level of CE release revealed that patients whose operations were done without CPB and had a CE release more than six times ULR seemed to have a significantly worse outcome both at 30 days and 1 year compared to the other three groups. This could partly explain the absence of a survival benefit in the OPCAB patients as a whole even though they have significantly lower CE release.
We can only speculate as to the reasons for comparatively higher mortality in the OPCAB patients with CE release greater than six times ULR. It could be that most of the high levels of CE release in this group was due to myocardial necrosis from perioperative ischaemia rather than from other sources giving rise to ‘spuriously high’ levels of CE release in patients undergoing ONCAB. This finding could corroborate recent reports that have suggested that although less commonly encountered following OPCAB, elevated troponin I release is more frequently associated with irreversible myocardial injury, as assessed by cardiac magnetic resonance imaging, than the more commonly encountered troponin I release following ONCAB surgery [9].
4.1 Study limitations
The major limitation of the present study is its retrospective nature and the nonrandomised allocation of surgical intervention technique. The treatment groups do have differing baseline characteristics and even though we used appropriate statistical methods for adjustment, this cannot categorically exclude subtle selective influences on our measured outcomes. As such we can only demonstrate association and not causality for our observations. Other limitations to the current study include the low number of deaths (41) in the OPCAB patients compared to 108 in the much larger ONCAB patient group. We did not make any systematic assessment of early or late graft patency among any of study subgroups and can therefore only speculate about the likely cause of high (≫6 × ULR) CE release that is more frequently caused by graft failure [24]. CK-MB values were not available for all patients, with 5% of them having only total CK values measured. The CK-MB values were not measured in this small proportion of patients because the total CK values themselves were very small, and considering the fact that CK-MB is usually released in proportion to total CK release, the CK-MB values in these cases would be well within the reference range. Moreover, after restricting the study to only patients who had CK-MB values measured, the findings of the study remained unchanged. It could be argued that a single measure of CE on the morning after their operation may not reflect the peak CE levels which might have been reached. However, in this study, the emphasis is not on a specific value of CE release, rather it is a comparison of CE release between two techniques. The CE levels were measured at around the same duration after the operation (12–18 h) for patients undergoing surgery with or without cardiopulmonary bypass; hence, comparison of the measured values between the two groups should be possible. We did not use more cardio-specific markers of cardiac myocyte injury such troponin T or I, which although less widely accepted and used as markers of myocardial injury following cardiac surgery, are less likely to be raised by noncardiac injury and may have additional prognostic value over CK-MB measurements [25]. A final limitation is that we looked at all cause mortality to analyse survival data and were unable to discriminate between deaths from cardiac and noncardiac causes, and also did not have 1-year outcome data regarding graft patency and ventricular function.
4.2 Clinical implications
This study concludes that avoidance of cardiopulmonary bypass for surgical coronary revascularisation is associated with significantly lower perioperative release of cardiac enzymes. Overall this is not associated with better 30-day and 1-year survival for the patients revascularised without CPB. Although occurring less frequently, high cardiac enzyme release (≫6 times ULR) following OPCAB is associated with higher mortality over the first postoperative year compared to patients with similar cardiac enzyme release following ONCAB. Clinicians should be vigilant in patients with high CE release following OPCAB as this may predict poor outcome for the patient.
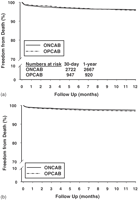
(a) Observed survival following coronary artery bypass surgery with and without the use of cardiopulmonary bypass. (b) Adjusted survival following coronary artery bypass surgery with and without cardiopulmonary bypass. (Adjusted for age, sex, body mass index, previous cardiac surgery, left ventricular ejection fraction, left main stem stenosis, number of major coronary arteries with stenosis ≫ 70%, priority of surgery, hypertension, hypercholesterolemia, peripheral vascular disease, diabetes, renal dysfunction, respiratory disease, preoperative white cell count and number of grafts.)
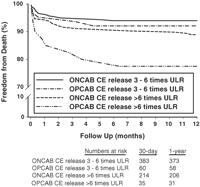
Survival following coronary artery bypass surgery in patients operated with and without cardiopulmonary bypass and with cardiac enzyme release three to six times ULR and more than six times ULR.
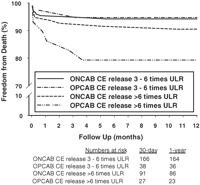
Survival following coronary artery bypass surgery in propensity-matched patients operated with and without cardiopulmonary bypass and with cardiac enzyme release three to six times ULR and more than six times ULR.

Table 1. Patient characteristics based on procedure performed

Table 2. Percentage of patients with CE release three to six times and more than six times ULR based on procedure performed (with odds ratios)
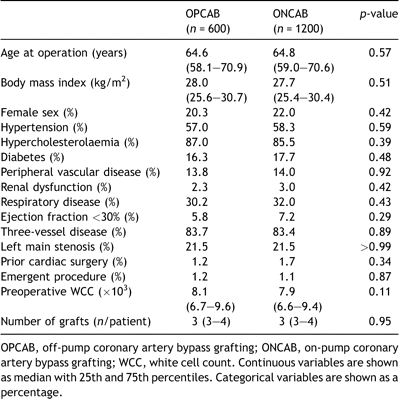
Table 3. Patient characteristics in propensity-matched groups
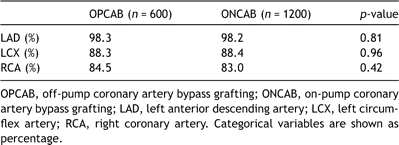
Table 4. Territories of myocardium revascularised in propensity-matched patients
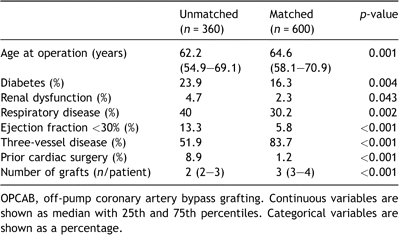
Table 5. Statistically significant differences between unmatched and propensity-matched OPCAB patients
We would like to acknowledge the co-operation given to us by all the Consultant Cardiac Surgeons at the Cardiothoracic Centre-Liverpool: Mr John A.C. Chalmers, Mr Walid C. Dihmis, Miss Elaine M. Griffiths, Mr Neeraj K. Mediratta, Mr D. Mark Pullan and Mr Abbas Rashid. We would also like to thank Miss Janet Deane, who maintains the quality and ensures completeness of data collected in our Cardiac Surgery Registry.
References




