-
PDF
- Split View
-
Views
-
Cite
Cite
Heyman Luckraz, Mike B Gravenor, Ravi George, Sue Taylor, Andrew Williams, Saeed Ashraf, Vincenzo Argano, Aprim Youhana, Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 5, May 2005, Pages 906–909, https://doi.org/10.1016/j.ejcts.2005.01.057
Close - Share Icon Share
Abstract
Objective: The development of acute renal failure following cardiac surgery is a rare but devastating complication with high morbidity and mortality. This study aimed to assess the incidence of acute renal failure necessitating continuous renal replacement therapy (CRRT) in patients who required cardiopulmonary bypass, to determine the factors associated with mortality and to evaluate long-term outcome. Methods: Patients who underwent cardiac surgery between October 1997 and 2003 and treated with CRRT were included (n=98). Six patients were then excluded (already in established renal failure pre-operatively) and one patient lost to follow-up. A retrospective analysis was carried out. Results: Overall CRRT was used in 2.9% (92/3172). The mean (SD) age of patients was 68 (10) years. Their mean pre-operative creatinine level and duration of cardiopulmonary bypass were 154 (87)micromol/l and 160 (84)min, respectively. Mean duration from surgery to establishment of CRRT was 50 (42)h. Mean creatinine level prior to hospital discharge was 168 (93)micromol/l. Thirty-day mortality was 42%. Significant risk factors for death were complex procedures (odds ratio=9.9), gastro-intestinal complications (OR=7.2), cross-clamp time over 88min (OR=5.9), re-exploration (OR=4.0) and patients age over 75 years (OR=3.3). Actuarial 1 and 5-year survivals (95% CI) were 53 (43, 63) % and 52 (42, 62) %, respectively. Only 2 (2.2%) patients required long term renal support. Conclusions: Acute renal failure necessitating the use of CRRT is a rare but serious complication post cardiopulmonary bypass. In the long-term, surviving patients are not likely to require further renal support.
1 Introduction
Acute renal failure (ARF) necessitating the use of continuous renal replacement therapy (CRRT) is a rare but devastating complication of cardiac surgery. Its incidence is reported to be to vary from 2 to 15% [1] with an associated mortality of 40–80% [2]. The risk factors predisposing to this complication include pre-operative renal dysfunction, age, vascular disease, emergency surgery and re-operations [3]. Moreover, Chertow et al. have shown that ARF is an independent predictor of mortality in cardiac surgery [4].
ARF is linked to multiple post-operative complications leading to prolonged hospitalisation and increased costs.
However, for patients who survive this complication, the need for long term renal replacement therapy is rare compared to patients who develop acute renal failure from other causes [5].
We reviewed the need for CRRT in our population group and assessed the morbidity and mortality in the short and long term.
2 Methods
All patients who underwent cardiac surgical procedures (from Oct 1997 to Oct 2003) and developed ARF with the need for CRRT were initially included in this study (n=98). Six patients were then excluded as they were in established chronic renal failure pre-operatively and were being haemofiltered. One patient was lost to long-term follow-up.
Data were retrospectively collected from patients' records and their intensive care management charts. Patients' status (alive or dead) was first confirmed using the hospital-based electronic database. Patients who were alive, were contacted through a telephone call to get an update on their renal condition. One patient was not contactable having moved out of the region.
The need for haemofiltration was confirmed by the renal physician and the indications included fluid management, acidosis and/or hyperkalaemia. The CRRT was instituted by the renal nursing staff. Vascular access was via a central vein with a dual lumen polyurethane catheter (Joka Kathetertechnik, 72379, Hechingen, Germany). Three Braun Trio pumps (Schwarzenbergen, Melsungen, Germany) were used. The dialysate fluid used (Monosol, Monosol K: Baxter, Thetford, UK, and Hemosol BO: Hospal International, Marketing Management S.N.C, Lyon, France) depended on the patients' electrolytes. Filtration was achieved through a Fresenius polysulfone filter (ultraflux AV 400S, 0.75m2, Fresenius Medical care AG, Badmonberg). Patients were anticoagulated with heparin maintaining an ACT (activated clotting time) of 180–200s. According to nomenclature for CRRT described by Bellomo et al. [6], the principle method of solute removal was by diffusion.
3 Statistics
Data were analysed using the SPSS and R statistical systems. Results are expressed as mean (SD), median (IQR) or percentages with 95% confidence intervals, where appropriate. Risk factors for fatal outcome were calculated using binary logistic regression. Data were available for the following potential risk factors: gender, age, ejection fraction, myocardial infarction, diabetes mellitus, hypertension, peripheral vascular disease, pre-operative creatinine levels, redo-surgery, operation type, operation timing, cardiopulmonary bypass and cross clamp times, re-exploration for bleeding, inotropic support, sepsis, wound infection, chest infection, the need for tracheostomy, gastro-intestinal and neurological complications. A final model for risk factors was obtained using a forward stepwise selection procedure. At each step the factor that lead to the greatest change in deviance for the model fit (and significant at the 5% level) was added to the model. Factors were retained in the model at subsequent steps if they remained significant at least at the 10% level, combined with an odds ratio of at least 2. Care was taken to examine the contribution of variables together, when a high degree of correlation was known between them (for example cardiopulmonary bypass and cross clamp times and operation type). Lastly, all 2-way interactions between variables in the final model were examined. All odds ratios reported below are adjusted for the factors in the final model. Survival analysis was carried out with the Kaplan–Meier survival curve.
4 Results
Overall CRRT was used in 2.9% (92/3172) of patients undergoing cardiac surgery.
The mean age of the patients was 68 (10) years with 67% being males. Peripheral vascular disease was diagnosed in 9% of patients and 13% underwent redo-surgery. The left ventricular function was impaired (EF≪50%) in 64% while in only 27% the surgery was carried out electively with 48% being urgent and 25% emergencies.
The mean pre-operative creatinine level was 154 (87)micromol/l. Isolated coronary artery bypass surgery (CABG) was performed in 34% of patients and CABG was combined with valvular surgery in 34%. Mean duration of cardiopulmonary bypass was 160 (84)min. The creatinine level prior to establishment of haemofiltration was 295 (89)micromol/l. The mean duration from surgery to haemofiltration was 50 (42)h and the renal replacement therapy was used for 6.4 (5.5) days.
Sepsis developed in 43% of patients during their ICU stay. A tracheostomy for assisting prolonged ventilatory support was performed in 32% and 40% required mechanical inotropic support with the intra-aortic balloon pump. Neurological and gastrointestinal complications were recorded in 14% and 16% of patients, respectively.
The median stay in intensive care was 10 (6, 18) days. The 30-day mortality was 42%.
Median in-hospital stay for the survivors was 21 (15, 37) days.
The mean creatinine level prior to hospital discharge was 168 (93)micromol/l. Only 2 (2.2%) patients required long term renal replacement therapy.
Significant risk factors for death (Table 1) were complex (redo, CABG+Valve, double valve, aortic surgery) procedures (OR=9.9), gastro-intestinal complications (OR=7.2), cross-clamp time over 88min (OR=5.9), re-exploration for bleeding (OR=4.0) and patients age over 75 years (OR=3.3). There was an indication that the effect of cross-clamp time on mortality was greater for patients with gastrointestinal complications.
Actuarial 1 and 5-year survivals were 53 (43, 63)% and 52 (42, 62)%, respectively (Fig. 1).
5 Comment
Acute renal failure post cardiac surgery remains a dreaded complication of this surgical procedure. Historically, its incidence is reported to be around 5% with 2% needing renal replacement therapy [7]. The latter was introduced as a mode of treatment for acute renal failure in the 1980s [8]. Intermittent use of haemofiltration in the acute post operative setting is associated with higher mortality (≫65%) [9] when compared to the continuous mode. The latter offers continuous and steady removal of fluid and toxins thus minimising the uraemic pro-inflammatory and immunologic effects [10].
Several mechanisms have been postulated for the occurrence of ARF. Hypo-perfusion of the renal medulla seems to be the most likely mechanism leading to ARF. This pathological process is due to vasoconstriction of the renal arterioles leading to redistribution of blood flow within the kidneys with under-perfusion in certain areas [11]. Reduction in renal blood flow has been demonstrated to be worse prior to rather than during CPB [12]. Prolonged renal arteriolar vasoconstriction is perpetuated by hypovolaemia, dehydration, increased levels of vasoconstrictors (vasopressin, angiotensin, aldosterone, cathecolamines, thromboxanes) as well as a decrease in atrial natriuretic peptide (ANP) and nitric oxide (NO) levels [13]. Moreover, renal damage may be precipitated when the kidneys are exposed to nephrotoxic agents (aminoglycosides, vancomycin, contrast dye) in the immediate pre-operative period. In the heart transplant setting, calcineurin inhibitors have been implicated in the development of renal damage [14]. Other factors which may play a role in the development of ARF post CPB include tissue oedema [15], micro-embolisation [16], endothelial dysfunction [17] and the generalised inflammatory response as a consequence of the CPB circuit [18].
The incidence of ARF has not decreased despite attempts to reverse the patho-physiological processes involved. The most important preventive step includes adequate re-hydration (normovolaemia) and maintenance of acceptable cardiac output.
When renal failure is established, fluid management, hyperkalaemia and acidosis remain the main indications for haemofiltration. The end result is an improvement in cardiac contractility which has been reported to be due to the elimination of myocardial depressant factor and cytokines [19]. This is better achieved by continuous renal replacement therapy than intermittent haemodialysis.
Lango et al. reported an 86% functional recovery but a 30% in-hospital mortality with high-volume (50–80ml/min) continuous renal replacement therapy [19]. Bent et al. reported a 40% mortality with early and intensive continuous renal replacement therapy (2000ml/h) [10]. The mean duration for CRRT was 3.98 (3.00, 4.97) days. There is evidence to suggest that a lower mortality is achievable with CRRT use if it is instituted early. An 80% mortality was reported when continuous renal replacement therapy was instituted over a week post-operatively [20]. In our patient group, the renal replacement therapy was instituted within 2 days of the surgical procedure and the filtration rate was set at 1000ml/h. The in-hospital mortality was 42% despite the setting of an older population group (mean age 68 years) and sicker patients (impaired LV function in 64% and elective surgery in only 27%). Similar experience was reported by Elahi et al. [21].
The long-term outlook in patients who survive this serious complication is relatively very good. Only two patients (2.2%) required long term continuous renal replacement therapy. In a prospective observational study in Scotland, Metcalfe et al., reported an ARF (all causes) incidence of 375 patients per million [5] with an 90-day mortality of 73.5 and 23.5% becoming dependent of renal replacement therapy due to end-stage renal dysfunction (ESRD). In the post CPB setting, few patients end up with ESRD probably reflecting the reversibility of the damage to the kidneys. Moreover, the long term survival (5 years) was as good as the early survival rate (1 year).
6 Conclusion
Renal replacement therapy (CVVHF) was needed in a small percentage of patients undergoing cardiac surgery. Their use was associated with a high morbidity and mortality. However, the long term outlook was excellent in patients who survived this onslaught.
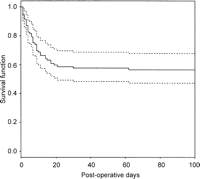
Estimated survivorship curve (with 95% confidence limits) for cardiac surgery patients following acute renal failure. Most deaths occurred during the first 20 days post-operation, with the 75% survival time being 7 days (4, 14).

Risk factors for fatal outcome. Odds ratios (adjusted for all factors) obtained from final logistic regression model (see text for details)




