-
PDF
- Split View
-
Views
-
Cite
Cite
Rosemary Radley Smith, Jo Wray, Asghar Khaghani, Magdi Yacoub, Ten year survival after paediatric heart transplantation: a single centre experience, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 5, May 2005, Pages 790–794, https://doi.org/10.1016/j.ejcts.2004.12.058
Close - Share Icon Share
Abstract
Objective: To report 10 years survival in children under the age of 16 years undergoing heart transplantation in a single institution. Methods: One hundred and thirty nine/one hundred and ninety three patients (73%) survived more than 1 year after transplant. Seventy four (53%) of these survived more than 10 (10.0–20.1) years. Age at operation was 10 days–15.5 (mean 8.1) years. Patients were maintained on ciclosporin and azathiaprine alone. Routine steroids only given to 4 patients for either persistent rejection or deteriorating renal function. Rejection diagnosed on clinical or echocardiographic grounds. No routine biopsies were performed. Bi-annual coronary angiography was used to diagnose graft coronary disease. Results: Graft coronary disease was found in 8 patients (11%), 2 were re-transplanted and have survived 4.3–7.2 years since. Two patients are alive without intervention 2.0–13.0 years from initial diagnosis. Two patients have undergone interventional procedures 11 and 16 years after transplantation and are alive 3 and 4 years, respectively, later. Seven patients have had post transplant lymphoproliferative disease (PTLD) and 6 have had no recurrence for 3–13 years after treatment. Impaired renal function with abnormal serum creatinine levels is increasingly common-11 patients have developed end stage renal failure, 7 requiring renal transplantation, hypertension occurred in only 3 patients other than those in renal failure. Late rejection episodes associated with probable non-adherence occurred in 7 patients. There were 10 late deaths; 2 from graft coronary disease; 1 from PTLD; 3 from renal failure; 3 from acute rejection and 1 from infection. Conditional actuarial survival from 1 year post transplant was 76 and 67% at 10 and 15 years, respectively. Conclusions: Survival for more than 10 years is increasingly realistic. In this age group adherence and deteriorating renal function are major challenges.
1 Introduction
Short-term survival after paediatric heart transplantation has improved significantly in the last decade. Initially early survival was approximately 75% but currently this survival has increased to almost 90% [1]. Reasons for this improvement include better selection of patients, improved donor organ preservation, modifications in surgical technique and advances in perioperative intensive care. The changes in early survival have resulted in better long-term survival, but this is illusory as the survival curves remain parallel irrespective of the era of transplantation [1]. With the excellent short-term results, prolonged survival has led to the appearance of long-term complications. If there are to be further improvements in long-term survival, it is essential that there is meaningful analysis of survival and risk factors for death, including complications related to long-term immunosuppression, differences in immunosuppressive regimes, late graft function and quality of life.
We report here the results of children under the age of 16 years undergoing heart transplantation at Harefield Hospital who have survived for more than 10 years since their transplant.
2 Patients and methods
Between August 1984 and April 2001, 193 children under the age of 16 years have undergone heart transplantation. One hundred and thirty nine patients (73%) have survived for more than 1 year since transplantation and 74 (53%) of these have been followed up for more than 10 years (10.0–20.1 (mean 15.9) years). Thirty-one patients have been followed up for more than 15 years. At the time of transplant, patients were aged between 10 days and 15.5 (mean 8.1) years (Fig. 1). Sixteen patients had congenital heart disease; 2 patients had Kawasaki disease and 56 patients had cardiomyopathy of whom 5 had hypertrophic cardiomyopathy; 6 restrictive cardiomyopathy and 45 the dilated form. Of the patients with dilated cardiomyopathy, in 9 there was a positive family history, 7 patients were transplanted for either active or old myocarditis, 2 patients had Adriamycin toxicity and 2 patients were transplanted for muscular dystrophy. Seventy patients underwent orthotopic heart transplantation and 4 underwent heterotopic transplantation because of elevated pulmonary artery pressure. The age of the donors was between 10 days and 24 (mean 8.8) years. Fourteen of these donors were the so-called domino heart donation from patients undergoing heart lung transplantation for primary lung disease.
2.1 Immunosuppression
Prior to 1987 all patients received induction therapy with anti thyomocyte globulin in the first 10 days and oral steroids which were tailed off and stopped after 3 months. Since 1987 no patient has received any induction therapy, apart from one dose of methylprednisolone 10mg/kg in the operating room when coming off bypass. Long-term immunosuppression was ciclosporin and azathiaprine based. In the first year after transplant levels were maintained between 150 and 300ng/ml, after the first post operative transplant year ciclosporin trough levels were maintained between 80 and 180ng/ml. Ciclosporin levels were monitored prior to 1994 by the EMIT method and since 1994 by LC-MS/MS (parent compound in whole blood by mass spectrometry). Azathioprine doses were maintained between 0–2mg/kg depending on the white blood cell count. If this fell below 5000 the azathiaprine dose was reduced and if it was below 3500 azathiaprine was stopped completely and not restarted even if the white blood cell count returned to normal, unless the patient had rejection episodes. Since 1987, routine steroids have only been given to 4 patients for either persistent rejection in 3, or deteriorating renal function in 1. Ten patients in the last 4 years have been changed to tacrolimus, 4 for recurrent rejection and 6 because of the side effects of ciclosporin. No patient received adjunctive therapy with statins.
Patients were routinely evaluated as an outpatient on a 3–6 monthly basis with clinical examination, electrocardiography and echocardiography, together with blood analysis including full blood count, urea, electrolytes, liver function tests and trough ciclosporin levels. Clinic visits were kept to a minimum compatible with safety and were tailored to avoid unnecessary interruption to schooling, etc. Biopsies were not routinely performed at any stage in the patient's post transplant follow-up. Rejection was diagnosed on clinical or echocardiographic grounds. Clinical grounds included malaise, fever, non-specific flu-like symptoms and in older patients the development of unexplained abdominal pain or nausea and vomiting, together with the appearance of a gallop rhythm. Echocardiographic indicators of rejection included a fall from previous baseline systolic function, increase in wall thickness and the appearance of a pericardial effusion. A rejection episode was defined as a clinical event, prompting acute augmentation of immunosuppressive therapy. Late rejection was defined as any rejection episode occurring more than 1 year after transplantation. Biopsies were not performed to confirm rejection. Patients underwent routine cardiac catheterisation with measurement of left and right heart pressures, left ventricular angiography and selective coronary angiography one year after transplantation and then bi-annually unless graft coronary disease had previously been detected or patients were being maintained on steroids. In these cases it was performed more frequently. Coronary angiograms were reviewed by at least two independent operators on each occasion. One of these operators has been the same person throughout the programme and has reviewed all the coronary angiograms.
Standard Kaplan-Myeier techniques were used to analyse survival data.
3 Results
Seventy-four patients have been followed up for 1178.2 (mean 15.9) patient years. At 10 years after transplant, 72 of the survivors were asymptomatic and in Class 1 NYHA, attending either school, university or in employment. The 2 remaining patients had neurological damage acquired at the time of transplantation. Somatic growth was normal in all patients.
At catheterisation right heart pressures were normal in all, including the 4 patients who had received heterotopic transplantation because of elevated pulmonary artery resistance. Left ventricular end diastolic pressure was normal in all patients except in 6 patients who had wide spread coronary artery disease on angiography.
4 Rejection and graft coronary artery disease
Rejection episodes occurring more than 1 year after transplantation were extremely uncommon. Late rejection occurring more than 10 years after transplantation occurred in 7 patients. In 2 this was the first rejection episode ever documented and both these children died. There was evidence of probable non-adherence to their immunosuppressive regime in 6/7 patients.
Graft coronary artery disease occurred in 8 (11%) patients. This was diagnosed angiographically in all 8 patients. No unexpected coronary artery disease was found on routine post mortem in patients dying of other causes. Two patients underwent successful re-transplantation 6.5 and 9.8 years after their original transplant and have survived 4.3 and 7.2 years, respectively, without recurrence of graft coronary artery disease. One patient in whom coronary disease was diagnosed 6 years after transplantation underwent successful coronary artery bypass grafting 16 years post transplant and remains well 4 years later. Another patient underwent coronary artery stenting 11 years after transplant and is also alive 3 years later. One patient was diagnosed as having mild coronary disease at 6 years and remains well with little progression of the disease, 13 years later. A further patient was diagnosed as having coronary artery disease at 12 years after the transplant and died 2 years later of an unrelated cause. Two patients died with known severe coronary disease 13.2 and 15.4 years after transplantation. Neither of these patients were considered suitable for re-transplantation because of high levels of Panel Reactive Antibodies in one and non-compliance in the other. Both children who required re-transplantation had a history of recurrent rejection episodes in the first year after transplant, and 2 of the other patients had a late rejection episode related to non-compliance. The other 4 patients with coronary artery disease all had a history of more than 2 rejection episodes. Coronary artery disease has not been found in any patient in whom there has been no history of rejection.
5 Post transplant lymphoprolifterative disease
Post-transplant lymphoproliferative disease (PTLD) has occurred in 7 patients. Six had B Cell lymphoma, which occurred within the first year after transplantation in 3 patients, all of whom responded to reduction in immunosuppression. Two of these patients subsequently had a recurrence of PTLD 6 and 10 years later which again responded to reduction in immunosuppression. All 3 patients with early PTLD are free of their disease 3–13 years after their last reduction in immunosuppression. Three additional patients developed PTLD more than three years after transplantation. All 3 required chemotherapy and 2 have survived free of disease 7–13.2 years after treatment. One patient who developed PTLD 13 years after transplant has died. One patient developed Hodgkins lymphoma 5 years after transplantation for which he received chemotherapy and he is well and disease-free 9 years later.
6 Renal function
There has been an increasing rise in serum creatinine levels with time (Fig. 2). Twelve patients all more than 11 years after transplantation currently have creatinine levels above our laboratory's normal value of 130mmol/lt. Eleven patients have developed end stage renal failure (ESRF) requiring either chronic ambulatory peritoneal dialysis or haemodialysis 7–18.8 years after their transplant. Seven of these patients have undergone renal transplantation 8–17.1 years after their cardiac transplant. One patient who developed renal failure 18.8 years after his cardiac transplant is currently on the waiting list for cadaveric renal transplantation. Three patients have died of renal failure 12.9–14.8 years following their original transplant, 2 of these patients were not considered suitable for renal transplantation. Hypertension requiring treatment with anti hypertensive drugs only occurred in 3 patients who did not have end stage renal failure.
7 Late deaths
There were 10 late deaths. Two patients died of graft coronary disease 13.2 and 15.4 years after transplant. One patient died of post transplant lymphoproliferative disease 12.9 years after his transplant. Three patients died from renal failure 12.9–14.8 years after their transplant. Three patients died from acute rejection 12.2–13.7 years after transplantation. This was related to non-adherence in all 3. One patient with neurological damage died from pneumonia 15.4 years after his transplant. The causes of late death in the patients in the study group more than 10 years after transplantation were similar to the causes of death in those patients dying between 1 and 5 years and 5 and 10 years after transplant, although infection was more common in the earlier cohort of patients and renal failure more common in the patients in this study group (Fig. 3).
8 Survival
Conditional actuarial survival in patients surviving more than 1 year after transplantation was 76% at 10 years, 67% at 15 years and 63% at 20 years (Fig. 4).
9 Discussion
In light of the experience from our adult programme which had been running for 4 years before we started transplanting children and who at that time were maintained without obvious increase in rejection episodes on minimal or no steroids, it was decided to avoid the routine use of steroids in children if at all possible. The complications of steroids in children are well known and include growth retardation, increased susceptibility to infections and hyperlipaedemia among other major problems, all of which may have an impact on both life expectancy and quality of life in transplanted children. It was decided in 1987 not to give steroids as induction therapy because of the problems which had also been encountered in other paediatric centres, in weaning some patients once started on steroids, off them. We also stopped at that time giving antithymocyte globulin at that time, because of problems with both supply and the cost. The incidence of rejection within the first year of transplantation and thereafter did not increase after cessation of induction therapy. Only four patients in the entire programme have required long-term steroid therapy.
Although late rejection was rare in our patients, when it did occur, the consequences were serious both in terms of poor survival and for the development of graft coronary artery disease. These findings have also been reported from large multi-centre studies from North America [2,3]. In these studies, risk for late rejection was associated with older age at transplantation and Black race, but in our patients the major risk for this complication was non-adherence. This has been reported with other solid organ transplants in children, [4,5] and has been found to increase with time [6]. In a recent study from our own institution we found that about one quarter of all patients were unintentionally non-adherent in that they forgot to take their medications on occasions, but that there was a significant, but smaller number of patients who were deliberately non-adherent [7]. A similar trend was seen in the patients in this study.
Graft coronary artery disease is the commonest cause for late death in adult heart transplant patients [8] and continues to be the leading cause of late death in children [1]. In our series only 11% of the patients had graft coronary artery disease 10–20 years after transplantation. No patient developed this complication unless they had had more than 2 rejection episodes. Recurrent rejection, particularly occurring in the first year after transplantation has been shown to be associated with coronary artery disease [9]. The relatively low incidence of graft coronary artery disease in our patients as compared with other series [10] could be explained by the fact that we use a steroid-free maintenance regime. Routine use of prednisolone has been shown to lead to an increased incidence of graft coronary artery disease [1]. A low incidence of this has been reported by other centres who also do not use steroids routinely [11]. Another contributory factor to the lower occurrence of this complication could be that all our donors were young, the oldest being only 24 years old. It would therefore, be unlikely that any of these organs would have had intrinsic coronary disease when transplanted.
The incidence of post transplant lymphoproliferative disease (PTLD) is similar to other series of heart transplant paediatric patients [12]. Because most children are sero-negative to the Ebstein–Barr virus (EBV) at the time of transplantation, it is not surprising that the incidence of PTLD is higher than in adults, [13] particularly if they receive a sero-positive donor. Treatment strategies are evolving [14] and vaccination against EBV may be available in the near future.
Hypertension in our patients was uncommon, with only 3 patients, other than those with chronic moderately-severe renal impairment required treatment. This low incidence may well be related to our steroid-free immunosuppressive regime. The association between use of steroids together with calcineurin inhibitors and hypertension is well documented [1].
Chronic renal failure in solid organ transplantation in adults is increasingly being reported [15,16]. The use of calcineurin inhibitors is known to be a major contributory cause [17] and there appears to be no difference in renal function between ciclosporin and tacrolimus-based immunosuppression [18]. Late reduction in the dose of either drug does not appear to improve renal function [19]. A recent study from Toronto has shown that in children the incidence of renal dysfunction in the first few years after transplantation is not high [20]. This is in contrast to other series [18,11] that have shown a marked decline in renal function leading to end-stage renal failure (ESRF) and renal transplantation. In our series, formal glomerular filtration rate was not measured but 11 patients had serum creatinine levels above our laboratory's normal value 11 or more years after transplantation. In addition 8 patients with ESRF have been listed for or have had a renal transplant and 3 other patients have died of renal failure. This is a major concern. It is possible that the decline in renal function may be related to early higher levels of ciclosporin that we used as has been seen in other studies [21]. We are addressing the problem by careful monitoring of ciclosporin levels and dosage without compromising the graft. In the adult transplant programme in our Institution we have had encouraging early results with a reduction in the rate of decline in renal function by changing patients to a calcineurin-free regime and using sirolimus and one patient in this study group has recently been changed to this regime.
Although the perioperative mortality in patients with congenital heart disease was significantly higher than the cardiomyopathy group, the mortality in both groups up to 10 years was similar as was the percentage surviving to that time. All the ten deaths that occurred more than 10 years after transplant were in the cardiomyopathy group. There were no deaths after 10 years in the smaller group of patients with congenital heart disease. The significance of this is unclear.
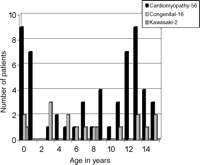
Age and diagnosis of patients who have survived more than 10 years.
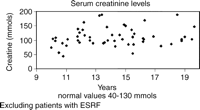
Serum creatinine levels at last follow-up. ESRF, end stage renal failure.
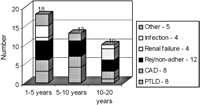
Causes of late deaths in patients follow-up for 1–5 years (column 1) 5–10 years (column 2) and for more than 10 years (the study group) (column 3). CAD, graft coronary artery disease, PTLD, post transplant lymphoproliferative disease.
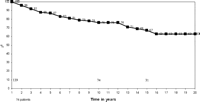
Conditional actuarial survival for patients under the age of 16 years who have survived for more than 1 year from transplant.
Appendix A. Conference discussion
Dr W. Davies (Rochester, MN, USA): I'm interested in your choice not to use a statin. There is some early evidence now that statins have effect on circulating progenitor cells and may affect graft atherosclerosis. Do you have any comments on whether you would review that policy?
Dr Radley-Smith: Some of these patients have been followed for 20 years, long before statins were used. Graft coronary disease is relatively uncommon. Only 11% of these patients had graft coronary disease, and some of those had developed it before the 10-year period.
In patients with known graft disease, we do use statins and perhaps I should have made that clearer, but we don't routinely use them. I am resistant from the adherence point of view of putting these young teenagers and adolescents on too many drugs.
Dr R. Deac (Targu-Mures, Romania): I have only one question. I haven't seen in your causes of death sudden death. Did you encounter such a sudden death in your group?
Dr Radley-Smith: Only associated with rejection, but not from graft failure. The other deaths were patients who were known to have coronary artery disease, known to have renal failure, known to have lymphoproliferative disease. And the only sudden deaths were in patients who came in with catastrophic rejection.
Dr G. Laufer (Innsbruck, Austria): Do you have any patients on calcineurin inhibitor-free protocol? I mean, did you try, with the availability of rapamycin or everolimus and MMF, to switch them because of increasing nephrotoxicity to such a protocol?
Dr Radley-Smith: We have recently started switching the patients. In our adult program, they have quite a lot of experience; but I have only one patient who was changed a week ago. A lot of these patients had developed their renal failure before people were happy to switch them to calcineurin-free treatments. But that is our policy to change the ones with deteriorating renal function in the future.
Presented at the joint 18th Annual Meeting of the European Association for Cardio-thoracic Surgery and the 12th Annual Meeting of the European Society of Thoracic Surgeons, Leipzig, Germany, September 12-15, 2004.
References
- coronary angiography
- hypertension
- heart transplantation, pediatric
- heart transplantation
- echocardiography
- renal transplantation
- renal function
- kidney failure, chronic
- biopsy
- kidney failure
- child
- lymphoproliferative disorders
- rejection (psychology)
- steroids
- tissue transplants
- infections
- cyclosporine
- diagnosis
- transplantation
- persistence
- coronary heart disease
- posttransplant lymphoproliferative disorder
- renal impairment
- creatinine tests, serum




