-
PDF
- Split View
-
Views
-
Cite
Cite
Manoj Kuduvalli, Aung Y. Oo, Nick Newall, Antony D. Grayson, Mark Jackson, Michael J. Desmond, Brian M. Fabri, Abbas Rashid, Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 4, April 2005, Pages 592–598, https://doi.org/10.1016/j.ejcts.2005.01.030
Close - Share Icon Share
Abstract
Objective: The purpose of this study was to examine the effect of peri-operative red blood cell (RBC) transfusion on 30-day and 1-year mortality following coronary artery bypass grafting (CABG). Methods: We retrospectively analysed 3024 consecutive patients who underwent isolated CABG between January 1999 and December 2001. Patient records were linked to the National Strategic Tracing Service, which records all mortality in the UK. Thirty-day and 1-year mortality were derived from Kaplan–Meier curves. Confounding variables were controlled for by constructing a propensity score for the probability of receiving a transfusion from core patient characteristics including the lowest recorded laboratory haemoglobin (LL Hb) from a clinical chemistry database (C statistic 0.81). The propensity score and the comparison variable (transfusion versus no transfusion) were included in a Cox proportional hazards analysis, allowing calculation of adjusted hazard ratios (HR) and Kaplan–Meier survival curves. Results: Nine hundred and forty (31.1%) patients received RBC transfusion during or within 72h of surgery. Predictors of the need for transfusion were LL Hb and lower body mass index, use of cardiopulmonary bypass, female sex, number of grafts, renal dysfunction, increased age, extent of disease, and prior CABG; these factors were all included in the propensity score. After 1-year of follow-up, 122 (4.03%) deaths occurred. The crude HR for 1-year mortality in patients transfused was 3.0 (P≪0.001). After adjusting for the propensity score, re-operation for bleeding, peri-operative blood loss and post-operative complications, the adjusted 30-day mortality was 1.9% in transfused patients compared to 1.1% in patients not transfused (P≪0.05). The adjusted HR for 1-year mortality in patients transfused was 1.88 (P≪0.01). Conclusions: Peri-operative RBC transfusion after CABG is associated with an increased risk of mortality during a 1-year follow-up period, with a large proportion of deaths occurring within 30-days.
1 Introduction
The argument for peri-operative red blood cell (RBC) transfusion assumes that: (a) adverse outcomes result from diminished oxygen-carrying capacity and (b) RBC transfusions, by enhancing oxygen-carrying capacity, can prevent these adverse outcomes [1]. The ‘triggers’ for RBC transfusion vary widely between institutions and between surgeons. The risks of blood transfusions still exist in the form of transmission of viruses such as Hepatitis B, Hepatitis C, Hepatitis G, HIV, HTLV I and II, haemolytic reactions (the incidence of fatal reactions still being around 1 in 250,000), and transfusion related acute lung injury (TRALI) occurring in 1 in 5000 transfusions. There is also some evidence suggesting an increased rate of recurrence in some malignancies, and bacterial contamination with organisms such as Staphylococcus epidermidis. Particularly in the United Kingdom, the possibility that variant Creutzfeldt–Jakob disease (vCJD) might be transmitted by transfusion is a cause for serious concern to transfusion services, and measures to mitigate this unknown risk dominate the blood safety agenda [2].
It is generally believed that patients undergoing cardiac operations have a lower margin of safety for tolerance of low haemoglobin levels. However, there is not much evidence to substantiate this belief. There are no real time monitors of oxygen supply and demand to the microcirculation of the whole body or individual organs. The ‘trigger’ for transfusion is usually a particular haemoglobin level that is of discomfort to the prescribing physician, and this is not defined by clear physiological parameters [3]. Transfusion decisions are made mostly on the basis of past teaching and inherited practices rather than any evidence base. In their study, Surgenor and colleagues [4] suggested that the specific hospital significantly affects RBC and component transfusion practice in coronary artery bypass graft (CABG) surgery, and this is attributed to institutional differences that, through reasons of training or hierarchy, become ingrained in hospitals. Various articles in the literature demonstrate that peri-operative transfusions in patients undergoing heart operations may actually be related to increased in-hospital morbidity and mortality. Blood transfusions have been linked to post-operative renal dysfunction [5], pneumonia [6], wound infections [7], severe sepsis [8] and hospital mortality [9]. There has been a suggestion recently that the adverse effects of peri-operative blood transfusion in patients undergoing CABG are not limited to the immediate post-operative period, but may extend further, with influences on long-term survival [10].
The aim of this study was to analyse the effect of peri-operative RBC transfusions on 30 day and 1-year mortality after CABG in our centre, while taking into account patient and disease characteristics.
2 Methods
2.1 Patient population and data
We performed a retrospective study on a total of 3024 consecutive patients undergoing isolated CABG surgery between 1st January 1999 and 31st December 2001 at the Cardiothoracic Centre-Liverpool. Patients undergoing CABG that was combined with a heart valve repair or replacement, resection of a ventricular aneurysm or other surgical procedures were not included. Definitions and data collection methods have been previously published [11] and are also available from www.nwheartaudit.nhs.uk
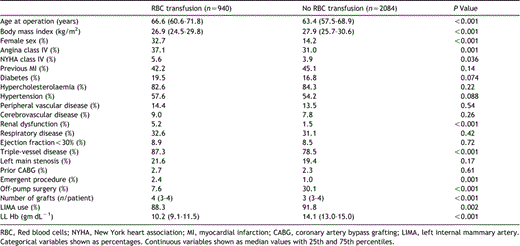
Data were collected prospectively during the patient's admission as part of routine clinical practice on the variables listed in Table 1. The lowest recorded laboratory haemoglobin (LL Hb) was taken as the lowest haemoglobin value measured by the clinical chemistry laboratory for a patient during his or her hospital stay after arrival in the intensive care unit, and this was abstracted from their computerised archives. Post-operative data on acute renal failure, myocardial infarction, stroke, and deep sternal wound infection were also collected. Post-operative stroke was defined as a new focal neurological deficit and comatose states occurring post-operatively that persisted for ≫24h after its onset and was noted before discharge. We excluded confused states, transient events and intellectual impairment from our study to avoid any subjective bias. Acute renal failure was defined as patients requiring dialysis. Deep sternal wound infection was defined in accord with the published evidence-based guidelines by the Centres for Disease Control and Prevention. Post-operative myocardial infarction was defined as a new Q-wave post-operatively in two or more contiguous leads on an electrocardiogram or significant rise in post-operative cardiac enzymes combined with haemodynamic and echocardiographic signs of myocardial infarction.
2.2 Red blood cell requirements
Blood transfusion data are provided routinely on a monthly basis by the local blood transfusion service. These data consisted of date of request for RBCs and number of units used during the patient's hospital admission. For the purposes of this study, the number of units used, if any, was calculated by summation across the patient's peri-operative period (during or within 72h of surgery) [12].
2.3 Patient follow-up
Patient records were linked to the National Strategic Tracing Service (NSTS), which records all deaths in the United Kingdom. To establish vital status at 1-year after operation, patients were matched to the NSTS based on patient name, National Health Service number, date of birth, gender and postcode.
2.4 Statistical methods
Due to non-normality of data, continuous variables are shown as median with 25th and 75th percentiles. Categorical variables are shown as a percentage. Comparisons were made with Wilcoxon rank sum tests and χ2 tests as appropriate. Deaths occurring as a function of time were described using the product limit methodology of Kaplan and Meier [13]. Treatment selection bias was controlled for by constructing a propensity score, otherwise known as a balancing score [14]. The propensity score was the probability of receiving a RBC transfusion, and was constructed from core patient characteristics and the LL Hb using forward stepwise logistic regression (C statistic=0.81). Once the propensity score was constructed for each patient, it was then included along with the comparison variable (transfusion versus no transfusion) in a Cox proportional hazards analysis [15], allowing calculation of adjusted hazard ratios (HR) with 95% confidence intervals (CI) and Kaplan–Meier survival curves. The Cox proportional hazards analysis also included the amount of blood loss in the intensive care unit (ICU) and the need for re-exploration for bleeding, as it was felt that these two variables were crucial in determining the need for transfusion. Also included in the risk adjustment was post-operative occurrence of myocardial infarction, acute renal failure, stroke, and deep sternal wound infection. In all cases a P value less than 0.05 was considered significant. All statistical analysis was performed retrospectively with SAS for Windows Version 8.2.
3 Results
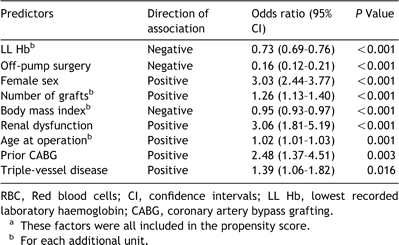
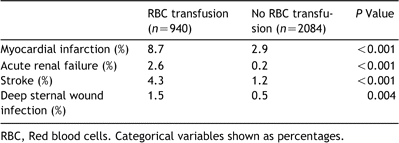
Nine hundred and forty (31.1%) patients received RBC transfusion during or within 72h of surgery. Twenty-eight patients who were not transfused within the peri-operative period, but received blood products after post-operative day three, were included in the non-transfusion group; median 1 RBC unit transfused (25th and 75th percentiles: 1–3). A comparison of patient characteristics based on whether they were transfused or not is shown in Table 1. Predictors of the need for transfusion are shown in Table 2; these factors were all included in the propensity score, which was used to risk-adjust the survival curves. Post-operative complications are shown in Table 3.
After 1-year of follow-up, 122 (4.03%) deaths occurred. The crude HR for 1-year mortality in patients transfused was 3.0 (95% CI 2.13–4.49; P≪0.001). Kaplan–Meier survival curves with number of patients at risk are shown in Fig. 1.
After adjustment with the propensity score, amount of blood loss, re-exploration for bleeding, myocardial infarction, acute renal failure, stroke, and deep sternal wound infection, the adjusted HR for 1-year mortality in patients transfused was 1.88 (95% CI 1.23–3.00; P≪0.01). The adjusted Kaplan–Meier survival curves are shown in Fig. 2. After excluding patients who died within 30-days after CABG, the adjusted HR for 1-year mortality in patients transfused was 1.67 (95% CI 1.01–2.89; P=0.049). The adjusted Kaplan–Meier survival curves with 30-day mortality excluded are shown in Fig. 3.
Figs. 4 and 5 examine survival in four different sub-groups based on LL Hb and whether the patients were transfused or not. Both the sub-groups of patients transfused received similar number of RBC units per patient (median 2 (25th–75th percentile 1–3)). LL Hb values had a significant impact on survival at 30-days in both groups, with the best outcome in patients not transfused with haemoglobin ≫10gmdL−1 and being the worst in patients transfused with haemoglobin ≪10gmdL−1 (Fig. 4, 70% CI is not over-lapping at 30-days). However, once the deaths at 30 days are excluded from the analysis, the LL Hb level does not appear to have a significant impact on survival at 1-year in patients who received a transfusion (Fig. 5, 70% CI over-lap for Groups C and D). However, it is important to note that the survival figures for both sub-groups of patients receiving transfusions in Fig. 5 are significantly lower than for either sub-group of patients who were not transfused.
4 Discussion
There is a definite need for more evidence-based decision-making with respect to peri-operative RBC transfusions. Apart from the fact that the blood transfusion is still associated with significant complications, the recent evidence demonstrating the potential adverse influence of peri-operative RBC transfusions on long term survival after CABG [10] is interesting, and alarming at the same time.
This study demonstrates that patients who did not receive a peri-operative RBC transfusion had a lower 30-day as well as 1-year mortality as a group, irrespective of their recorded LL Hb. Survival advantage at 1-year was demonstrated even after excluding deaths occurring within 30 days of operation from the survival analysis. Engoren and colleagues [10] demonstrated a similar survival benefit over 5 years in patients undergoing CABG who did not receive peri-operative transfusions. However, they did not include haemoglobin values in their analysis. There are studies in the literature that suggest that haemoglobin concentration after cardiopulmonary bypass may influence morbidity and mortality. One study [16] concluded that low haemoglobin concentrations in the immediate post-operative period increase post-operative complications significantly and that subsequent correction of anaemia by red cell transfusion did not modify at least the renal and abdominal complications. This suggests that early prevention of a decrease in haemoglobin concentration would appear preferable to late treatment with transfusions. This hypothesis is borne out in our study. Amongst the patients who received a peri-operative transfusion, the ones with LL Hb of greater than 10gmdL−1 had a lower 30-day mortality compared to those who had LL Hb of less than 10gmdL−1. However, Hardy and colleagues [16] were unable to determine whether it was the low haemoglobin, or the associated blood transfusions, which led to the increased post-operative complications. Our study shows that even amongst the patients with LL Hb less than 10gmdL−1, those that were not transfused had a lower 30 day and 1-year mortality than the ones that were given peri-operative RBC transfusions. Hence, one might infer that blood transfusion may have contributed to the adverse outcomes.
Another study published in 1998, showed that a high haematocrit of ≫34% (corresponding approximately to a haemoglobin level of 11gmdL−1) on arrival in the ICU was associated with an increased rate of myocardial infarction and was an independent predictor of infarction [17]. They concluded on the basis of this that there is no rationale for transfusion to an arbitrary level after CABG. However, they also demonstrated that all cause mortality in patients with low and high haematocrit on arrival in the ICU was similar, even though the group with low haematocrit had a significantly higher incidence of RBC transfusion. In our experience, the lowest peri-operative mortality was seen in patients with LL Hb greater than 10gmdL−1 and not receiving a peri-operative transfusion and the highest peri-operative mortality was seen in the group transfused with LL Hb less than 10gmdL−1. We are unable to explain this difference in our findings. However, the study referred to was not specifically designed to analyse the effect RBC transfusion might have on peri-operative mortality.
On the other hand, our findings concur with Michalopoulos and colleagues [9], who showed an independent association of blood transfusions with hospital mortality after CABG. Another study [18] suggested that poorer outcomes in women undergoing CABG might be associated with greater need for transfusion during operation.
There is evidence from a randomised control study to show that lowering the haemoglobin threshold for transfusion in coronary artery bypass procedures does not adversely affect patient outcome [19]. This fact is further reinforced by our study in which patients with LL Hb less than 10gmdL−1 fared better if they were not transfused than if they were, both at 30 days and 1-year follow-up. Even in the non-cardiac surgical setting, there is evidence to show that a restrictive strategy of RBC transfusion with the use of a threshold as low as haemoglobin of 7gmdL−1 as a trigger for transfusion, and maintaining haemoglobin between 7.0 and 9.0gmdL−1 is at least as effective as, and possibly superior to a liberal transfusion strategy in critically ill normovolaemic patients [20].
The evidence in favour of RBC transfusion to maintain a certain level of haemoglobin is sparse. Two database studies have shown low haematocrit on cardiopulmonary bypass to be associated with mortality and morbidity after coronary artery surgery [21,22]. However, both of these studies have shown increased adverse outcomes with very low haematocrit value, i.e. 23% in the study by Defoe and colleagues, and 14 and 17% for low-risk and high-risk patients, respectively, in the study by Fang and colleagues. Furthermore, very low haematocrit is a marker for transfusion, and neither of these studies have examined transfusion as a variable in their analyses.
There are various ways in which transfusion can produce adverse outcomes. Stored bank blood has major performance limitations in oxygen delivery due to severe depletion of 2,3 diphosphoglycerate. The oxygen dissociation curve is significantly shifted to the left, thus hampering oxygen delivery to the tissues. In theory, due to this, stored blood could draw oxygen out of the tissues in the microcirculation. The RBCs in stored blood are also very fragile and non-distensible. Furthermore, blood transfusion presents a large inflammatory stimulus and contributes further to the inflammatory state already present after cardiopulmonary bypass (CPB) [23]. Cytokine levels in post-CPB patients show a 15-fold rise with a single blood transfusion [24].
This study shows that the best outcomes are in patients who have LL Hb greater than 10gmdL−1 and do not receive a peri-operative transfusion. This stresses the need to use various strategies for blood conservation and to minimize the blood loss. It does not support the strategy to transfuse red cells in order to maintain an arbitrary ‘comfortable’ value of haemoglobin concentration. Pharmacological strategies that decrease peri-operative blood loss in cardiac surgery, in particular aprotinin and lysine analogues also decrease mortality, the need for re-thoracotomy and the proportion of patients receiving a blood transfusion [25].
As mentioned in the methods of the study, LL Hb was taken as the lowest haemoglobin value measured by the clinical chemistry laboratory for a patient during his or her hospital stay after arrival in the ICU. We realise that there may be some patients in the group that was transfused who may have had even lower haemoglobin levels measured either during the course of surgery, or in the ICU along with routine blood gas analysis, which may have been the triggers for transfusion. These values would not have been recorded in the clinical chemistry laboratory database. Nevertheless, the values of LL Hb that have been used in our study are still the strongest predictors of the need for transfusion, therefore justifying their inclusion in the analysis. Moreover, both the sub-groups of patients transfused received similar number of RBC units per patient (median 2 (25th–75th percentile 1–3); P=0.53) and there was no significant difference in the BMI of patients between the two sub-groups (27.0 versus 26.5kg/m2; P=0.23). Hence, even if lower haemoglobin values were measured and not recorded, their relationship to the recorded LL Hb levels in both the sub-groups of patients receiving transfusions are likely to be similar. This justifies using the recorded LL Hb for sub-group analysis in the patients receiving RBC transfusions. It could be argued that the LL Hb values in the transfused patients could actually be the haemoglobin values recorded after the correction of more severe anaemia, and it was the anaemia and not the transfusion that led to the poor outcomes. However, irrespective of the LL Hb levels, the transfused patients as a group had a higher 30 day and 1-year mortality compared to non-transfused patients. The peri-operative anaemia could possibly have resulted in the higher 30-day mortality figures, but it is difficult to explain how it could result in significantly reduced survival of the transfused patients at 1-year follow-up even after excluding patients dying within 30 days of operation from the analysis. Even if this were the case, we have shown that transfusing these possibly sicker and more anaemic patients did not favourably alter their adverse outcomes, and at best were ineffective transfusions. The fundamental question of whether it is the need for, or the effect of transfusion that results in adverse outcomes can only be answered in a randomised controlled study of liberal or conservative transfusion strategies among patients displaying no evidence of tissue hypo-perfusion and no symptoms, but having haemoglobin values in the ‘grey zones’ between 7 and 10gmdL−1.
There are various limitations to our study. Firstly, being a retrospective database study, by its nature, it is only capable of showing association between variables and outcomes, and is unable to demonstrate cause and effect. Furthermore, the retrospective nature of the study cannot account for uncollected or unknown variables affecting the outcome or transfusion bias that are not correlated strongly with the variables used in the risk adjustment. Another limitation of the study is that we did not have the data to differentiate between transfusions given during the operation, and those given post-operatively on the day of the procedure. Especially in patients whose operation is done on cardiopulmonary bypass, intra-operative transfusions are more likely to be triggered by haemoglobin or haematocrit values rather than clinical need, and adding this parameter to our analysis might have further strengthened our conclusions. A third limitation is that we used all-cause mortality as an end point, and were unable to differentiate between cardiac and non-cardiac causes of death. A fourth limitation is the fact that there was no strict transfusion policy post-operatively and the triggers for transfusion were dependent on the individual clinician treating the patient. A final limitation is the fact that we have divided our patients into two large groups based on LL Hb levels, rather than examine them in smaller sub-groups in an attempt to establish a reasonable trigger for transfusion that did not adversely affect the outcome. However, subdividing the patient population into smaller groups would have reduced the power of the study for analysis of outcome.
In conclusion, in this study, peri-operative RBC transfusion appears to be associated with an increased 30-day and 1-year mortality in patients undergoing coronary artery bypass grafting.
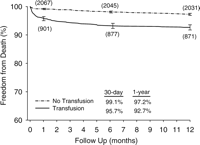
Observed survival following coronary artery bypass grafting (y-axis starts at 60%).
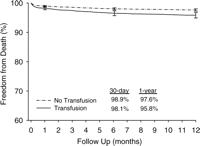
Risk-adjusted survival following coronary artery bypass grafting (y-axis starts at 60%).

Risk-adjusted survival following coronary artery bypass grafting (excluding 30-day mortality) (y-axis starts at 90%).
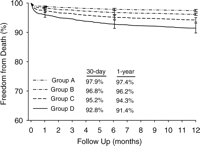
Risk-adjusted survival showing the relationship between RBC transfusion and LL Hb (y-axis starts at 60%). Group A, patients not transfused with LL Hb≫10gmdL−1; Group B, patients not transfused with LL Hb≪10gmdL−1; Group C, patients transfused with LL Hb≫10gmdL−1; Group D, patients transfused with LL Hb≪10gmdL−1.

Risk-adjusted survival showing the relationship between RBC transfusion and LL Hb (excluding 30-day mortality) (y-axis starts at 90%). Group A, patients not transfused with LL Hb≫10gmdL−1; Group B, patients not transfused with LL Hb≪10gmdL−1; Group C, patients transfused with LL Hb≫10gmdL−1; Group D, patients transfused with LL Hb≪10gmdL−1.
Appendix A. Conference discussion
Dr M. Deja (Katowice, Poland): Bearing in mind that your propensity scoring and the calculations are hopefully all safe and well performed, I understand that blood transfusion itself is causing mortality. So are you suggesting you should not transfuse these patients, or what is the practical outcome of this study?
Mr Kuduvalli: As you know, this is an observational study and this cannot demonstrate any causative effects, it can only demonstrate associations, and all I can say that this shows a very strong association between red cell transfusion itself and peri-operative and 1-year mortality after teasing out all the other variables as far as possible using propensity scores, which to my mind is probably the best way of comparing apples and oranges and making a comparison between like and like.
We are not making any recommendations because we don't have the power in this study to make any recommendations. What we are trying to highlight here is a very strong association, and we are also trying to suggest that there should be some caution before transfusing red cells and it should not be a knee-jerk reaction as in trying to maintain a supposedly comfortable level of haemoglobin for a patient.
The actual answer as to whether it is the red cell transfusion itself which is leading to the mortality will require a randomized study in patients who maybe have haemoglobins in the gray zones between 8 and 10 post-operatively and then randomizing them to two groups and so on and so forth, which is a very impractical thing to do. So I think with the data we have, this is the closest we can get to an answer.
Dr Deja: But are you suggesting that in some of these patients they would do better if we did not transfuse them?
Mr Kuduvalli: As I said initially right at the outset, there will always be patients where the red cell transfusions will be inevitable, and there are always clinical situations where red cell transfusions have to be made.
From the data which we have, what we can show is that transfusion itself, irrespective of the nadir hemoglobin of the patient, seems to have an adverse effect on the survival at 30 days and 1 year after these patients have had coronary artery bypass grafting, and as far as we could, we have tried to tease out all the other issues using the statistical analysis, which includes post-operative bleeding as well as take back for bleeding for re-exploration.
Dr G. Mani (New Delhi, India): Were these packed cells leuko-reduced or not? Did you have any evidence whether the packed cells which were administered to the patients were leuko-reduced or not?
Mr Kuduvalli: No, these were not leuko-depleted. They were just packed cells.
Dr Mani: That makes a difference.
Dr D. Harris (Cape Town, South Africa): You didn't elaborate what is the cause of the deaths in the patients. I think that is an important question to ask.
Mr Kuduvalli: This is one of the weaknesses of the study in the sense that we have looked at all causes of mortality at 1 year. We have not been able to tease out only cardiac mortality in these patients. So we don't know the exact cause of death. Does that answer your question?
Dr Harris: Yes.
Presented at the joint 18th Annual Meeting of the European Association for Cardio-thoracic Surgery and the 12th Annual Meeting of the European Society of Thoracic Surgeons, Leipzig, Germany, September 12–15, 2004.
We would like to acknowledge the co-operation given to us by all the Consultant Cardiac Surgeons at the Cardiothoracic Centre-Liverpool: Mr JAC Chalmers, Mr WC Dihmis, Mr BM Fabri, Miss EM Griffiths, Mr N Mediratta, Mr DM Pullan, and Mr A Rashid. We would also like to thank Janet Deane, who maintains the quality and ensures completeness of data collected in our Cardiac Surgery Registry.




