-
PDF
- Split View
-
Views
-
Cite
Cite
Stanislav Ovroutski, Vladimir Alexi-Meskishvili, Brigitte Stiller, Peter Ewert, Hashim Abdul-Khaliq, Julia Lemmer, Peter E. Lange, Roland Hetzer, Paralysis of the phrenic nerve as a risk factor for suboptimal Fontan hemodynamics, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 4, April 2005, Pages 561–565, https://doi.org/10.1016/j.ejcts.2004.12.044
Close - Share Icon Share
Abstract
Objective: The introduction of the Fontan operation for single ventricle physiology was based on the dual principle of the pulmonary blood flow. It is postulated that normal breathing movements are necessary for passive blood flow into the lungs. We compared patients with and without palsy of the phrenic nerve regarding the sufficiency of Fontan hemodynamics. Methods: We analyzed 85 consecutive patients, who were available for follow-up after completion of their total cavopulmonary connection (TCPC) between February 1992 and February 2003. The median age at TCPC completion was 4.3 (range 1.3–37) years. Sixty were operated on with an extracardiac conduit and 25 with a lateral tunnel. Fifty patients underwent postoperative heart catheterization with contrast angiography. The diagnosis of diaphragm paralysis was made using echocardiography, fluoroscopy and X-ray examination. Surgical diaphragm plication was performed in 13 patients (Four before and nine after Fontan operation) at a median of 2.2 years after the diagnosis. Results: Twenty-one patients developed fixed palsy of the phrenic nerve during a total of 225 operations before and including completion of TCPC. There were no differences in the incidence of phrenic nerve paralysis between small children (aged ≪3 years) and older patients or between patients with the extracardiac and intracardiac Fontan procedures. There were no differences in the duration of mechanical ventilation. However, prolonged pleural effusions and a hospital stay of longer than 2 weeks were noted more frequently in patients with palsy (P≪0.05). During the median follow-up of 4.6 (range: 0.7–11.4) years significantly more patients with phrenic nerve palsy developed chronic ascites compared to those without palsy (8 of 20 vs. 2 of 65; P≪0.001). Conclusions: Phrenic nerve palsy was recognized as a risk factor for suboptimal Fontan hemodynamics due to the hindrance of passive venous blood flow. Patients with phrenic nerve palsy have a longer hospital stay and a higher incidence of prolonged pleural effusions and of chronic ascites, than those without. Early diaphragm plication may be favorable to optimize the Fontan circuit in these patients. Completion of the TCPC in patients with diaphragm paralysis should be viewed critically.
1 Introduction
Rodbard and Wagner demonstrated in their animal studies in 1949 the possibility of sufficient pulmonary circulation without pump function of the right ventricle and carried the ideas of William Harvey and Magendie from the 16th and 19th centuries forward into clinical medicine [1]. Further clinical surgical experience in the 20th century confirmed that the Fontan operation for surgical palliation of single ventricle physiology could be introduced based on the dual principle of the pulmonary blood flow [2–4]. It is clinically evident that normal breathing movements and negative intrathoracic pressure are necessary for passive blood flow into the lungs [1,5,6]. For optimization of the blood flow within the cavopulmonary connection and avoidance of turbulence, different modifications of the Fontan operation have been proposed [7,8]. Despite the slight differences in flow pattern in vitro, the extracardiac conduit and lateral tunnel are the approaches most often used for the cavopulmonary connection; the hemodynamic outcome is comparable with both approaches [9,10]. In the course of the staged surgical procedure towards the Fontan operation children underwent multiple operations with the risk of damage of the phrenic nerve, which leads to postoperative dysfunctional breathing movements. Therefore we compared early and long-term results in patients with and without palsy of the phrenic nerve regarding the sufficiency of Fontan hemodynamics after the lateral tunnel and extracardiac conduit operation.
2 Patients and methods
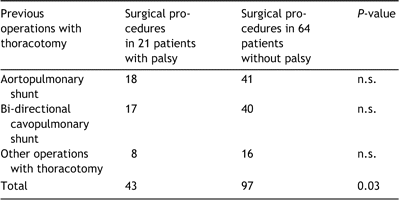
Eighty-five consecutive patients operated on between February 1992 and February 2003 in our institution (60 with extracardaic conduit and 25 with lateral tunnel) were available for the follow up. At the time of Fontan operation the patients had a median age of 4.5 years (range 1.3–37.0 years) and a median body weight of 16.0kg (range 8.3–70kg). The gender distribution was 42 males and 43 females. The main cardiac diagnoses were tricuspid atresia (n=27), double inlet left ventricle (n=20), unbalanced double outlet right ventricle (n=6), pulmonary atresia with intact interventricular septum (n=6) unbalanced atrioventricular septal defect (n=4), mitral atresia (n=4), and other complex forms of the single ventricle (n=18). At least one operation for aortopulmonary shunt (a total of 59 shunts) was performed in 42 patients (49%). Staged Fontan procedure with previous bi-directional cavopulmonary shunt was carried out in 57 patients (67%), more before extracardiac than lateral tunnel modification (47 vs. 10, P=0.001). A total of 225 operations, including 140 previous operations and 85 Fontan operations as listed in Table 1, were performed in the group.
During follow-up all patients underwent at least one echocardiographic examination per year. The diagnosis of diaphragm paralysis was made using ultrasound, fluoroscopy and X-ray examination. Fifty-one patients underwent postoperative heart catheterization with contrast angiography. The central venous pressure, mean pulmonary artery pressure in the left and right pulmonary arteries and the arterial oxygen saturation were compared between patients with and without palsy of the phrenic nerve. Further, the clinical data, especially on the development of chronic or recurrent ascites, were subjected to comparative analysis.
Surgical diaphragm plication was performed in 13 patients (four before and nine after Fontan operation) at a median age of 2.2 (range 0.1–11.4) years after the diagnosis (Fig. 1). A ‘central pleating’ technique according to the method described by Shoemaker et al. [11] was used in all patients.
3 Results
Twenty-one patients developed fixed palsy of the phrenic nerve during a total of 225 operations before and including completion of total cavopulmonary connection (9.3%). In eight patients one-sided diaphragm paralysis was known before Fontan operation. In four of them diaphragm plication was performed preoperatively and the mean PAP before TCPC was low with a median of 11.5 (range: 5–13) mmHg without statistical differences from the other patients.
3.1 Early postoperatively
After Fontan operation a total of six patients had left-sided diaphragm paralysis and 13 right-sided paralysis. Two patients developed bi-lateral diaphragm palsy. There were no differences in the incidence of phrenic nerve paralysis between young children (aged ≪3 years at Fontan operation, n=20) and older patients or between the extracardiac and intracardiac Fontan procedures. Otherwise the incidence of left-sided phrenic nerve palsy correlated significantly with repeated surgery on the left side of the thorax such as for coarctation, left aortopulmonary shunt or left superior caval vein to left pulmonary artery anastomosis (there were five patients with left-sided palsy out of 22 patients with left-sided surgery, compared to three out of 63 other patients, P=0.025). There were no differences in the early postoperative course regarding the duration of mechanical ventilation; however, prolonged pleural effusions and a hospital stay of longer than 2 weeks were noted more frequently in patients with palsy (P=0.023). The early postoperative data are presented in Table 2.
Chest X-ray examination in anterio-posterior projection showed significant differences with the diaphragm elevated up to three intercostal spaces on the affected side compared with the non-paralytic side (Fig. 1).
3.2 Medium-term results
During the median follow-up of 4.6 (range: 0.7–11.4) years patients with phrenic nerve palsy developed chronic or recurrent ascites significantly more frequently than those without paralysis (8 of 21 vs. 2 of 64; P≪0.001).
Heart catheterization with angiography was performed at a median of 2.8 (0.2–11.4) years after Fontan operation. The mean pulmonary artery pressure at rest was higher in patients with palsy compared with those without palsy, but did not reach statistical significance (median of 10 vs. 12.5mmHg; P=0.14).
Slower contrast medium passage through the conduit or tunnel, retrograde flow into the hepatic veins and redistribution of the pulmonary flow away from the affected side were visualized in patients with phrenic nerve palsy.
In two patients with bilateral diaphragm paralysis very long storage of the contrast medium without normal effect on inspiration was observed. One of these patients, prematurely born, suffered from chronic pulmonary insufficiency with mean PAP of 16mmHg, despite bilateral diaphragm placation, and died 2 years after surgery.
In four patients with recurrent ascites and without elevated pulmonary artery pressure (6–12mmHg) who underwent post-Fontan diaphragm plication improvement of the hemodynamics with disappearance of ascites was noted.
4 Discussion
In this study phrenic nerve palsy was recognized as a significant risk factor for the development of postoperative complications, including late ascites as a sign of suboptimal Fontan hemodynamics due to insufficient passive venous blood flow. Diaphragm paralysis remains a cause of severe postoperative morbidity. An incidence of postoperative diaphragm paralysis of up to 12% has been reported in the literature [12–15]. Patients with complex cardiac malformations who need multiple re-operations are probably at high risk because of adhesions and complicated preparations. The incidence of diaphragm paralysis in patients who received TCPC was higher than in the total patient population (1.8%) operated on in our institution over the last decade, as described by Stiller et al. [16].
The phrenic nerve is very susceptible to damage, especially in small infants undergoing cardiac surgery, due to possible lesions caused by preparation, mechanical strain, contusion and impact of hypothermia or hyperthermia [13,17,18]. The necessity of repeated surgery in the vulnerable for modified Blalock-Taussing or aortopulmonary shunt, for bi-directional cavoplmonary shunt, enlargement of the pulmonary arteries or total cavopulmonary connection may be responsible for this damage [14,18,19].
Newborns and small infants are the patients most often affected, with diaphragm paralysis leading to delayed extubation or requiring early diaphragm plication [14–17]. The duration of mechanical ventilation in the current study in patients with phrenic nerve paralysis was, however, not longer than in those without, probably because older children (median age 2–4 years) who are candidates for the Fontan operation have a higher chance of being weaned from the respirator quickly and without early diaphragm placation due to better development of the breathing mechanics than in newborns and infants [12,14–16]. Despite successful weaning from the respirator, patients with Fontan circulation are still at high risk for delayed complications if diaphragm paralysis is present [5,6,12,16].
Children after Fontan operation, who have usually undergone multiple operations, have limited thoracic expansion and low muscular strength of the thoracic cage to compensate the absence of diaphragm movement [12]. Furthermore, more than 60% of the systemic venous flow in patients with Fontan circulation was measured during inspiration, and normal inspiration led to more than 24% flow increase [6,20].
Different authors have described how aggressive postoperative diaphragm plication could significantly decrease early morbidity such as prolonged mechanical ventilation or pleural effusions [11,12,15]. Matejka et al. [21] focused on the necessity of diaphragm plication only in cases of severe respiratory insufficiency. This indication was barely present in the current series.
Iverson and colleagues [22] described normalization of diaphragm function during 6–12 months, if no complete denervation occurred. Nevertheless, we would emphasize that early plication in Fontan patients, who are highly dependent on passive blood flow into the lungs, improves the Fontan circuit and may prevent the development of later complications. Better movement of the non-paretic side without negative impact of the ‘dancing’ mediastinum and without mediastinal shift to the contralateral side after diaphragm plication was clearly observed in our series using fluoroscopy. In cases of not critically elevated pulmonary artery pressure accompanied by good ventricular function the disappearance of ascites could be expected after diaphragm plication, as in four of our patients. However, in Fontan-patients with diaphragm palsy, since the sucking effect of the lung due to normal inspiration remains inadequate, further close-meshed follow-up is required [5,6]. If exact diagnosis early postoperatively seems to be difficult, fluoroscopy should be performed after extubation. Chronic or recurrent ascites and pleural effusions as signs of suboptimal Fontan hemodynamics were noted frequently in the patients with diaphragm palsy [12].
It is important to note that we observed the development of such symptoms not just early postoperatively but also during the mid-term and until late follow-up. The passive blood flow from the lower part of the body without augmentation due to negative pressure effect of the lungs, without pump function of the right ventricle and contrary to gravitation, results in a pressure increase within the cavopulmonary connection [5,6]. In our series, we observed elevated pressure within the Fontan pathway in patients with diaphragm paralysis although the TCPCs were performed with either lateral tunnel or extracardiac conduit, where optimal laminar blood flow is expected [7,9].
Elevated pulmonary artery pressure together with chronic heart failure are the main factors in the multifactorial causality of failing Fontan hemodynamics [23,24]. Normal breathing movements could probably support the forward flow from the inferior caval vein, even in children with elevated pulmonary artery pressure up to 16mmHg, which we observed in some of our patients. Otherwise the absence of diaphragm movement with elevation leads to reduction and shrinking of the lung on the affected side and hinders the Fontan circulation even in patients with normal PAP of under 12mmHg, as we observed in two of our children, both with good ventricular function. The unfavorable subdiaphragmatic venous hemodynamics with absent inspiratory flow augmentation and low transhepatic gradient may be responsible for chronic ascites in patients with phrenic nerve palsy after Fontan operation [25].
We would speculate that after primary successful total cavopulmonary connections patients with postoperative diaphragm paralysis are at high risk for failed hemodynamics as time goes on. Patients with severe failing Fontan hemodynamics mostly suffer from arterial desaturation, ascites and pleural effusions and are readmitted to the hospital repeatedly [12].
Therefore, we would postulate that, if the diagnosis of phrenic nerve palsy is confirmed, diaphragm plication should be done immediately after this and before discharge. In patients with low pulmonary artery pressure and good ventricular function who are otherwise good candidates for Fontan procedure, the plication should be done preoperatively.
5 Conclusions
Phrenic nerve palsy was recognized as a significant risk factor for insufficient Fontan hemodynamics due to the hindrance of passive venous blood flow. Patients with phrenic nerve palsy have a longer hospital stay and a higher incidence of prolonged pleural effusions and of chronic ascites than patients without palsy. Early diaphragm plication may be favorable to optimize the Fontan circuit in these patients. Completion of the TCPC in patients with diaphragm paralysis should be viewed critically.
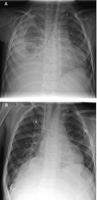
(A) Right-sided phrenic nerve paralysis in a 4.2-year-old boy with chronic ascites and pleural effusions 1 year after extracardiac conduit Fontan operation. The mean pulmonary artery pressure 9 months after surgery was 13mmHg. (B) Significant augmentation of the lung volume on the affected side after diaphragm plication. Disappearance of ascites and effusions was observed within the following weeks. Four years after ECFO the patient is doing very well and shows normal somatic development.

The authors would like to thank Anne M. Gale, ELS, of the Deutsches Herzzentrum Berlin for editorial assistance.
References
- angiogram
- ascites
- common ventricle
- fontan procedure
- hemodynamics
- vascular flow
- conduit implant
- pleural effusion
- lung
- cardiac catheterization
- inspiration
- child
- fluoroscopy
- follow-up
- paralysis
- phrenic nerve
- surgical procedures, operative
- diagnosis
- mechanical ventilation
- phrenic nerve paralysis
- plication of diaphragm
- total cavopulmonary connection
- venous flow




