-
PDF
- Split View
-
Views
-
Cite
Cite
Marc Ruel, Alexander Kulik, Buu K. Lam, Fraser D. Rubens, Paul J. Hendry, Roy G. Masters, Pierre Bédard, Thierry G. Mesana, Long-term outcomes of valve replacement with modern prostheses in young adults, European Journal of Cardio-Thoracic Surgery, Volume 27, Issue 3, March 2005, Pages 425–433, https://doi.org/10.1016/j.ejcts.2004.12.002
Close - Share Icon Share
Abstract
Objectives: To examine the multiple impacts of valve replacement on the lives of young adults. Methods: Patients(N=500) between age 18 and 50 who had aortic valve replacement (AVR) and/or mitral valve replacement (MVR) with contemporary prostheses were followed annually. Events, functional status, and quality of life were examined with regression models. Results: Median follow-up was 7.1±5.3 years(maximum 26.7 years). Five, 10, and 15-year survival was 92.7±1.7, 88.3±2.4 and 80.1±4.7% after AVR, and 93.1±2.3, 79.5±4.3 and 71.5±5.4% after MVR, respectively. Survival decreased with concomitant coronary disease (hazard ratio (HR): 4.5) and preoperative LV grade (HR: 2.0/grade increase) in AVR patients, and with atrial fibrillation (HR: 5.5), coronary disease (HR: 5.7), preoperative left atrial diameter (HR: 3.0/cm increase) and NYHA class (HR: 2.1/class increase) in MVR patients. Despite reoperation, late survival was equivalent between bioprostheses and mechanical valves in both implant positions. The ten-year cumulative incidence of embolic stroke was 6.3±2.4% for mechanical AVR patients, 6.4±2.9% for bioprosthetic AVR patients,12.7±3.9% for mechanical MVR patients, and 3.1±3.1% for bioprosthetic MVR patients. Atrial fibrillation (HR: 2.8) and smoking (HR: 4.0) were riskfactors for stroke in MVR patients. In AVR patients, SF-12 physical scores, freedom from recurrent heart failure, and freedom from disability were significantlyhigher in bioprosthetic than mechanical valve patients. Career or income limitations were more often subjectively linked to a mechanical prosthesis in both implantpositions. Conclusions: Late outcomes of modern prosthetic valves in young adults remain suboptimal. Bioprostheses deserve consideration in the aortic position, as mechanical valves are associated with lower physical capacity, a higher prevalence of disability, and poorer disease perception. Early surgical referral and atrial fibrillation surgery may improve survival after MVR.
1 Introduction
Prosthetic heart valve replacement in young adults is fraught with a myriad of potential problems over the remaining lifespan of the patient. Common complications include thromboembolism, bleeding,and reoperation [1]. Valve replacement in young adults entails a choice between a mechanical prosthesis with the risks and in convenience of lifelong anticoagulation versus a bioprosthesis with limited long-term durability necessitating eventual reoperation. Large series onthe impacts of valve replacement on the lives of young adults are lacking; we therefore examined vital, medical, physical, and psychosocial outcomes in a cohort ofyoung adult patients who underwent aortic and/or mitral valve replacement with modern mechanical and bioprostheticvalves.
2 Methods
2.1 Patients, procedures and follow-up
This study used prospectively collected data from patients aged 18 to 50 years who had first time aortic valve replacement (AVR), mitral valve replacement (MVR), or double valve replacement (DVR) at the Universityof Ottawa Heart Institute between 1976 and 2002 (N=837). Primary valve repair procedures were excluded from this study. In order for the results tobe generalizable to today's cardiovascular practice, the analyses were restricted to the subcohort of patients implanted with prostheses that were still available in North America and Europe as of this writing (N=500).
Patients were seen in a dedicated valve clinic six months postoperativelyand on an annual basis thereafter. Of the 500 patients, 30 were lost to follow-up at a mean of 2.2±1.2 years after valve replacement. The remainder of thecohort was followed for a mean of 7.9±5.0 years postoperatively (maximum follow-up time: 26.7 years).
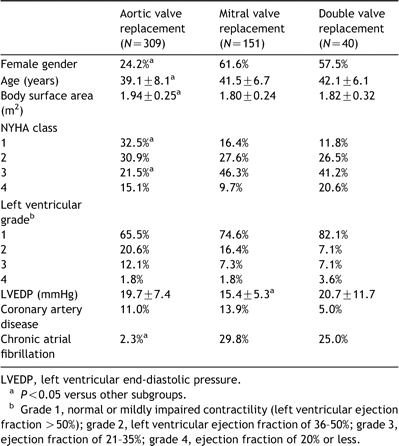
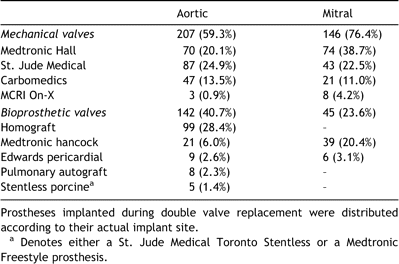
The preoperative characteristics of the cohortare presented in Table 1. The initial operation consisted of AVR in 309 patients (mechanical in 171, bioprostheticin 138), MVR in 151 patients (mechanical in 109, bioprosthetic in 42), and DVR in 40 patients (mechanical in 36, bioprosthetic in 4). Table 2 shows the types of prostheses implanted.
2.2 Anticoagulation
During the postoperative period,anticoagulated patients initially received heparin (2500 units IV q6h or 5000 units subcutaneously every 12h) until the International Normalized Ratio (INR) orprothrombin time was within therapeutic range. Oral anticoagulation was managed by the surgeon during the initial 3-month postoperative period and subsequently bythe primary care physician or cardiologist. Home anticoagulation, which was not routinely available in Canada during the study period, was not used. Patients with mechanical prostheses or with chronic atrial fibrillation were anticoagulated with warfarin according to guidelines in effect at the time, as previously described[2]. Briefly, these consisted for mechanical valves of a target prothrombin time or INR of 2.0–3.0×normal in patients with St. Jude, Medtronic Hall, Carbomedics or On-X aortic valves, and 2.5–3.5× normal in patients with St. Jude, Medtronic Hall, Carbomedics or On-X mitral valves. These target ranges were increased by 0.5× normal if atrial fibrillation or another indication for anticoagulation wasalso present, such as previous embolism or known thrombotic disorder. Patients with two mechanical valves were kept in the range of 3.0–3.5× normalprothrombin time or INR. The decision to add aspirin (80 or 81 mg daily) or dipyridamole (50–100 mg three times daily) to the anticoagulation regimen was leftto the surgeon, cardiologist, or primary care physician.
Warfarin anticoagulation was used at the discretion of the surgeon for a period of 3 months afteroperation in patients who underwent bioprosthetic valve replacement and who were in sinus rhythm. Warfarin was subsequently discontinued if sinus rhythm was maintained and no other indication for anticoagulation was present. Non-anticoagulated patients with a bioprosthetic valve were kept on 325 mg of aspirin dailyunless contraindicated, and the addition of dipyridamole was left to the decision of the patient's physician. Bioprosthetic valve patients in atrialfibrillation were anticoagulated at a prothrombin time or INR target range of 2.0–2.5× normal.
2.3 Outcomes
• Mortality after valve replacement was defined either as early/perioperative (i.e. in hospital or within 30 days of operation) orlate.
• Stroke was defined as the presence of a neurological deficit lasting more than 3 weeks [1,2]. Patients diagnosed with a stroke after 1986 had computerized tomography of the head. Strokes were characterized either asembolic or as an intracranial bleeding event based on the primary mechanism and computerized tomographic appearance of the lesion. The diagnosiswas confirmed by a neurologist or internist.
• Bleeding events were classified as major (i.e. requiring hospital admission ortransfusion, of intracranial location, or causing death), intracranial, or minor (i.e. prospectively recorded but notmajor).
• Reoperation was defined as any operation that repaired, altered, or replaced a previously operated valve [1,3]. The indication and details of reoperation were available in all patients, and the decision as to the choice of replacement devicewas individualized between patient and surgeon. Reoperation for structural valve deterioration (SVD) was defined as a reoperation performed primarily because of anintrinsic abnormality of the valve that caused stenosis or regurgitation, exclusive of infection or thrombosis. Reoperated patients were reclassified as such fromthe day of reoperation according to their new valve model and type, and continued to be followed in the clinic. Their follow-up period and events with the new prosthesis were analyzed separately from that pertaining to the previous prosthesis.
• Heart failure was defined as per previous publications as the composite end-point of (1) New York Heart Association (NYHA) functional class 3 or 4 for more than 4 consecutive weeks, corroborated with physicalexamination, chest X-Ray, ECG and echocardiography findings when available, or (2) death where the primary or main contributing diagnosis was heart failure[4].
• Other prosthesis-related complications were recorded according to the Guidelines forReporting Morbidity and Mortality after Cardiac Valvular Operations [1,5].
• Quality of life was determined with the physical (PCS) and mental (MCS) component scores of the short form (SF)-12 health status instrument, which was completed by phone during the year 2003, at a mean of 8.0±4.9 years after valve replacement. Information regarding adverse events and reoperation(s) to date, if applicable, were validated with the patient at the time of phone contact in addition to being separately available from the prospective clinical and echocardiographic follow-updatabases. Additional data were also obtained from the patient regarding: (1) current functional status by use of the New York Heart Association (NYHA) heart failure classification (supplementing the prospective record of NYHA class symptoms after valve replacement) [4];(2) employment status (including date(s) of change in status and the occurrence of permanent disability, defined as the inability to carry full-time work due tomedical reasons, if applicable); (3) disease perception (including the question ‘did your valve disease or prosthesis significantly affect your work, career,or income?’); (4) marital status (including date(s) of status change, if applicable); (5) mood (including the past or present occurrence of an affective disorder); and (6) the patient's overall satisfaction with the current prosthesis Disability, divorce, and past depression were dated and linked, in the event of reoperation(s) prior to the 2003 quality of life interviews, to the actual prosthesis in place at the time of occurrence of the event(s). Conversely, physical and mental component scores, disease perception, and patient satisfaction with the current prosthesis were linked, in cases where reoperation had already occurred, to the prosthesis in place at the time of the phone interview.
2.4 Statistical analyses
Data were imported andanalyzed in Intercooled Stata 8.0 (Stata, College Station, TX). Continuous data are presented as mean±standard deviation, except for survival and eventsrates which are reported as mean±standard error. For actuarial analyses of the various outcomes in this study, patients were censored at the time of their last follow-up visit or at the time of death if the outcome of interest had not occurred, and censoring was assumed to be independent of predictors and outcomes. For actual analyses, patients were considered not to have experienced a given outcome by a specific time point if either (1) the time point had been reached and the outcome had not occurred, or if (2) death took place before the time point had been reached and the outcome had not occurred before death. Analyses were performed on the entire cohort, between subcohorts of mechanical versus bioprosthetic valves, and within mechanical or bioprosthetic valves according to 4 subclasses of valve models (Table 3). These 4 subclasses consisted of: (1) stented bioprostheses (MedtronicHancock I, Medtronic Hancock II, or Edwards Perimount); (2) homografts (in the aortic position only); (3) tilting-disc mechanical valves (Medtronic Hall); and (4) bileaflet mechanical valves (St. Jude Medical, Carbomedics, or MCRI On-X).
2.4.1 Univariate analyses
Potential predictors of actuarial, actual, or continuous outcomes were individually tested for equality with a Log-Rank test, Fischer's exact test, or analysis of variance, respectively. Effect estimates and P values were used to identify confounding (arbitrarily defined as a 10% or more change in effect estimate resulting from entry of the additional term) and to determine eligibility for entry into multivariate regression models, respectively.
Adjusteda hazards of adverse outcomes, by prosthesis type and subclass
2.4.2 Multivariateanalyses
2.4.2.1 Cox proportional hazards models
Actuarial analyses incorporated variables that had a P value of 0.20 or lesson univariate Log-Rank testing in order to account for positive or negative confounding. No automated model selection procedure was used and all reportedvariables, unless collinear as arbitrarily determined by a Spearman's rank correlation coefficient ≥0.30 and a P value <0.005, were used simultaneously. In addition, biologically important covariates (such as atrial fibrillation or left ventricular grade) were forced into the models to account for confounding and are reported as such in the text.
2.4.2.2 Logistic regression models
Actual outcome analyses at specific timepoints and binary outcomes analyses were performed by incorporating variables that had a P value of 0.20 or less on univariate analysis in a logistic regression model. As for Cox proportional hazards models, biologically important covariates were forced into the logistic regression models and are reported in thetext.
3 Results
3.1 Survival
3.1.1 Early
Overall perioperative mortality was 16 of 500 patients (3.2%). This consisted of 6 of 309 patients who underwent AVR, 6 of 151 patients who underwent MVR, and 4 of 40 patients whounderwent DVR.
3.1.2 Long-term
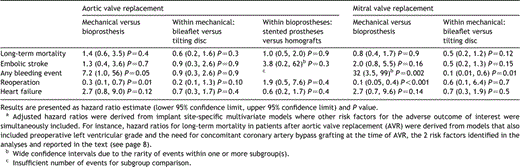
Fig. 1 displays the long-term survival of patientsin the cohort. The overall five, 10, and 15-year survival by implant site was 92.7±1.7, 88.3±2.4 and 80.1±4.7% after AVR, 93.1±2.3,79.5±4.3 and 71.5±5.4% after MVR, and 83.1±6.3, 78.9±7.2 and 69.1±11.2% after DVR, respectively. Long-term survival was significantly worse after double valve replacement than after either aortic or mitral replacement (HR: 2.2; 95% CI: 1.1, 4.5; P=0.03). On the otherhand, there was no significant difference in survival between patients who underwent AVR versus those who underwent MVR, no survival difference between patients implanted with a mechanical versus a bioprosthetic valve in either implant position, and within mechanical or bioprosthetic valves in either implant position there was no survival difference between the 4 subclasses of prostheses examined (Table3).
3.1.3 Risk factors
3.1.3.1 Aortic
Independent multivariate risk factors for decreased long-term survival in patients after AVR were preoperative left ventricular grade (HR: 2.0 per grade increase; 95% CI: 1.2, 3.6; P=0.02) and theneed for concomitant coronary artery bypass grafting at the time of AVR (HR: 4.5; 95% CI: 1.5, 13.1; P=0.007). Prosthesis type or subclass, age atthe time of surgery, atrial fibrillation, left atrial size, preoperative New York Heart Association (NYHA) heart failure class, and the type of prosthesis (i.e.mechanical versus bioprosthetic) had no significant effect.
3.1.3.2 Mitral
Independent risk factors for late death after MVR were preoperative atrial fibrillation (HR: 5.5; 95% CI: 1.5, 19.9; P=0.01), preoperative left atrial diameter (HR: 3.0 per cm increase; 95% CI: 1.0,9.1; P=0.04), preoperative NYHA class (HR: 2.1 per class increase; 95% CI: 1.1, 4.1; P=0.02), and the need for concomitant coronary artery bypass grafting at the time of MVR (HR: 5.7; 95% CI: 1.0, 32.3; P=0.05). Prosthesis type or subclass, age at the time of surgery, gender,left ventricular grade, and the type of prosthesis had no significant effect.
3.1.3.3 Double valve
In the limited number of young patients who underwent DVR in the cohort, the only independent risk factor for reduced survival was preoperative atrial fibrillation (HR: 7.2; 95% CI: 1.3, 41;P=0.03).
3.2 Stroke
3.2.1 Embolic
Four strokes occurred in the early postoperative period in the cohort; 2 involved AVR patients (0.6%) and 2 occurred in MVR patients (1.3%). In the chronic phase, the 10-year cumulative incidence oflate postoperative embolic stroke was 6.3±2.4% for mechanical AVR patients, 6.4±2.9% for bioprosthetic AVR patients, 12.7±3.9% for mechanical MVR patients, 3.1±3.1% for bioprosthetic MVR patients, and 16.1±9.4% for mechanical DVR patients. No strokes occurred in the 4 bioprosthetic DVR patients.
3.2.2 Risk factors
In the aortic position, there was no difference in embolic stroke risk between mechanical andbioprosthetic valves. In the mitral position, mechanical prostheses were a risk factor for stroke on univariate analysis (HR: 2.2±0.9;P=0.04); however, this lost significance on multivariate analysis as atrial fibrillation and smoking entered the model. The only independent risk factors for embolic stroke in MVR patients were atrial fibrillation (HR: 2.8; 95% CI: 1.1, 7.2; P=0.04) and smoking (HR: 4.0; 95% CI: 1.4, 11;P=0.008). No significant independent effect was observed with respect to prosthesis type or subclass (Table3). The addition of aspirin to warfarin anticoagulation had no significant effect on the incidence of embolic stroke in patients with mechanical valves, regardless of implant position or valve type.
3.2.3 Bleeding events
Three intracranial bleeding events occurred in thecohort during the follow-up period. There were an additional 26 patients who experienced bleeding at extracranial sites during the follow-up period, 12 of whom required hospital admission, and 6 for whom surgical intervention was necessary. All patients denoted above except two (one who had a spontaneously bleeding intracranial aneurysm and one who had a minor bleeding episode) were on warfarin anticoagulation. The linearized rates of intracranial, major, and minor bleeding events in anticoagulated patients from the cohort were respectively, 0.1% per year, 0.4% per year, and 0.9% per year.
The presence ofa mechanical prosthesis, non-dissociable from the use of warfarin in the series, was a significant risk factor for bleeding events in either implant position(Table 3). Within mechanical valves, tilting disc prostheses were also associated with significantly more bleeding events than bileaflet prostheses in the mitral position.
3.3 Reoperation
Reoperation took place in 8 mechanical aortic valvepatients, 11 aortic bioprosthesis valve patients, 3 mechanical mitral valve patients, and 20 mitral bioprosthesis patients. All patients reoperated for aortic ormitral mechanical valve failure, as well as patients reoperated for mitral bioprosthesis failure received a mechanical valve at reoperation. Although the decisionwas individualized, patients reoperated for aortic bioprosthesis failure more than 8 years after initial implantation received another bioprosthetic valve in theseries, while the remainder, including early failing homografts, were re-implanted with an aortic mechanical valve. The overall mortality associated with redo-AVRwas 6.0%; that of redo-MVR was 11.1%.
3.3.1 Bioprostheses
The actuarial and actual cumulative incidences of reoperation forbioprosthetic valves are shown on Fig. 2. Structural valve deterioration was the primary indication in 83% ofbioprosthetic valve reoperations. There was an opposite discrepancy between actuarial and actual reoperation rates in aortic versus mitral bioprosthesis patientsdue to lower long-term survival and longer follow-up in the mitral patients (Fig. 1).
Fig. 3 depicts the reoperation rates of aortic homografts versus aortic stented bioprostheses in the series. Stented bioprosthesesinitially fared better than homografts but demonstrated a higher failure rate from 10 years onward, with no significant difference overall in reoperation ratesbetween patients who received an aortic homograft and those who received a stented aortic bioprosthesis.
3.3.2 Risk factors
Smoking was identified as a potential risk factor for accelerated structural bioprosthesis deterioration, with a trend towards an increased risk ofreoperation in patients with bioprosthetic aortic as well as bioprosthetic mitral valves (HR: 8.3±10.2,P=0.09).
3.3.3 Mechanical valves
Mechanical valves had a cumulative incidence of reoperation of 5.4±2.2% at10 years in the aortic position and of 4.2±2.4% at 10 years in the mitral position. These rates correspond to the 15 year reoperation rates in this series,as no event led to the reoperation of a mechanical heart valve after 10 or more years had elapsed since the initial operation. No difference in reoperation rateswas observed between tilting disc and bileaflet prostheses in either implant position (Table3).
3.4 Heart failure
Heart failure appeared more frequent in patients implanted with a aortic mechanical valvethan in those with a aortic bioprosthesis (HR: 2.7; 95% CI: 0.8, 9.0; P=0.12), but the trend was not significant (Table 3). This relationship was independent of atrial fibrillation (HR: 18.6±32.6 for recurrent NYHA III or IV status;P=0.10), left ventricular grade (OR: 1.3±0.8 per grade increase; P=0.6), and aortic prosthesis size (OR: 0.7±0.2 persize increase; P=0.2). Although a similar trend was observed in MVR patients (Table 3), there was also no significant difference in the crude and adjusted incidence of recurrent heart failure symptoms between mechanical and bioprosthetic MVR and DVRpatients.
3.5 Quality of life
3.5.1 Short form-12 health status instrument
Fig. 4 shows the mean physical and mental component scores of the short form (SF)-12 health status instrument. Quality of life was good tovery good in all groups. Physical component scores for AVR patients were significantly higher in patients with aortic bioprostheses than in those with mechanicalaortic valves (by 9.9%; 95% CI: 2.7, 17.1; Bonferroni-adjusted P=0.02). This difference of aortic bioprostheses over mechanical valves was observed irrespectively of the use of homografts versus stented bioprostheses (Fig. 4A). There was no other significant difference in SF-12 physical or mental component scores between prosthesis types and subclasses in either implant site.
3.5.2 Employmentstatus
Disability was more prevalent in mechanical AVR than in bioprosthetic AVR patients (31.2 versus 21.7%, mechanical versus bioprosthesis AVR patients, respectively; adjusted OR: 2.8; 95% CI: 1.1, 7.3; P=0.03), despite adjusting for atrial fibrillation, age, left ventricular function, and coronary disease. This was not observed in MVR or DVR patients. The prevalence of disability was not different between patients implanted with an aortic homograft versus as tented bioprosthesis or, in either implant site, between tilting disc and bileaflet valve recipients. Disability either preceded or occurred within a year of surgery in 60.0% of disabled patients. In the remaining 40.0% of disabled patients, it occurred at a mean of 6.6±4.2 years postoperatively. Prosthesis type, subclass, or implant site did not significantly affect the time between the surgery to the occurrence of disability in patients in whom it occurred.
3.5.3 Disease perception
A higher proportion of AVR patients with a mechanical prosthesis answered ‘yes’ tothe question ‘did your valve disease or prosthesis significantly affect your work, career, or income?’ than bioprosthetic AVR patients (35.2 versus23.0%, mechanical versus bioprosthetic AVR patients, respectively; adjusted OR: 2.2; 95% CI: 1.02, 5.1; P=0.04). This difference was observed with the use of either aortic homografts or aortic stented bioprostheses, and was independent of atrial fibrillation, age, left ventricular function, and coronary artery disease. This relationship was also observed in mechanical versus bioprosthetic MVR patients (55.6 versus 18.2% answered ‘yes’, mechanicalversus bioprosthetic MVR patients, respectively; adjusted OR: 6.3; 95% CI: 1.1, 37; P=0.04), with no differences between valve subclasses.
3.5.4 Marital status
There was no difference in marital status between implant sites, prosthesis types and subclassesin the cohort, with a cumulative incidence of divorce of 32.7% at a mean age of 48.6±8.4 years at last follow-up. Valve replacement did not appear to constitute a major factor, as 75.5% of patients who experienced a divorce separated at a mean of 9.0±6.6 years before valve replacement. In only 24.6% ofdivorced patients did the separation occur after surgery, at a mean of 5.1±3.7 years postoperatively.
3.5.5 Mood
The reported cumulative incidence of depression in the cohort was 24.1%, with no difference according to prosthesis type or subclass, implant site, left ventricular function, atrial fibrillation, coronary disease, or age. The point prevalence of depression at latest follow-up was 9.8%, with no significant difference between subgroups.
3.5.6 Satisfaction with the prosthesis
In AVR patients, 30.3% of mechanical valve patients versus 20.2% of patients with a bioprosthesis either expressed dissatisfaction with their prosthesis or were uncertain whether they would choose the same prosthesis again (multivariateadjusted OR for prosthesis non-satisfaction: 1.8, mechanical versus bioprosthetic AVR, respectively; 95% CI: 0.8, 4.0; P=0.13). This relationship was not statistically significant, however, and the only independent predictor of non-satisfaction with the chosen aortic prosthesis was whether a patient had experienced reoperation (OR: 4.7; 95% CI: 1.0, 23; P=0.05). Within aortic mechanical valves, there was a trend towards patients implanted with atilting disc valve more often expressing dissatisfaction with the prosthesis than those who received a bileaflet prosthesis (OR: 2.4, 95% CI: 0.9, 6.3; tiltingdisc versus bileaflet; P=0.07). No predictor of dissatisfaction with the prosthesis(es) was identified in MVR and DVRpatients.
4 Discussion
Patients in need of heart valve replacement at a young age face a difficult situation, as anideal valve substitute does not yet exist. In this follow-up study of 500 patients operated between 18 and 50 years of age for aortic and/or mitral valvereplacement with modern prostheses, multivariate analyses indicated that: (1) long-term survival is equivalent between patients who underwent AVR and those who underwent MVR, but worse in those who had DVR, with no significant survival difference between patients implanted with a mechanical versus a bioprosthetic valve inany of the implant positions; (2) risk factors for decreased long-term survival include: preoperative left ventricular grade and the need for concomitant coronary artery bypass grafting in AVR patients; preoperative atrial fibrillation, preoperative left atrial diameter, preoperative NYHA class, and the need for concomitantcoronary artery bypass grafting in MVR patients; and atrial fibrillation in DVR patients; (3) the incidence of stroke and anticoagulation-related bleeding events in this younger patient cohort is approximately half that of a regular age cohort [2], with no difference in embolicstroke rates between mechanical and tissue valves; (4) smoking cessation and concomitant atrial fibrillation surgery may decrease the risk of stroke in affected young MVR patients, although the latter requires further investigation; (5) more than fifty percent of patients with a bioprosthetic valve, regardless of implantsite, are reoperated within 15 years, with smoking being a modifiable risk factor for accelerated deterioration in younger patients (as was also recently noted ina regular age cohort [3]); (6) physical capacity and freedom from disability are better in bioprosthetic AVR than inmechanical AVR patients; and (7) disease perception is better with bioprosthetic valves in either implant site.
While this follow-up study represents one ofthe largest series of young patients implanted with contemporary heart valve prostheses to date, it also illustrates the difficulties in examining thesepatients’ outcomes with adequate statistical power. Fewer young patients require valve replacement compared to older subjects, and event rates are lower than in regular valve patient cohorts. Young valve patients also constitute a relatively heterogeneous population compared to regular valve patients, with somepresenting with endocarditis and marginal lifestyle, Marfan's syndrome, rheumatic fever, premature coronary disease, congenital defects, etc…, whilethe remainder are generally healthy. Compliance issues add to this biologic heterogeneity, as age under 55 years is a known risk factor for poor compliance with warfarin therapy [6]. Consequently one should exercise caution at drawing strong conclusions on the choice of valvefor a given patient from the present study, and instead see these data as useful prognostic information for surgeons, cardiologists, and patients. For instance,these results refute the commonly held belief that mechanical AVR patients are at significantly greater embolic stroke risk than patients with a bioprostheticaortic valve. The study also illustrates the need for multicenter prospective studies and databases to better understand the important problem of valve replacementin the young adult. As the present data indicate, young patients with modern prosthetic valves continue to have a higher mortality risk than if they did not havevalve disease, indicating that this health issue remains far from resolved.
This results of this study suggest that aortic bioprostheses may constitute aviable alternative to mechanical valves in young patients in need of AVR who wish to avoid anticoagulation. Aortic bioprostheses, in the form of stentedbioprotheses or homografts (which have equivalent performance) appear to be associated with better physical capacity, social functioning, and prosthesis satisfaction. On the other hand, a bioprosthetic mitral valve offers no clear advantage compared to a mechanical MVR with the exception of warfarin avoidance (acrucial aspect for women contemplating childbirth), and may be actually more hazardous as late survival is poor and reoperation for redo-MVR carries significantrisk. Although a higher rate of embolic stroke is seen in mechanical MVR patients, this is confounded by the higher co-prevalence of atrial fibrillation, itself astroke risk. In the future, less thrombogenic mechanical prostheses, newer forms of anticoagulation, and the routine performance of concomitant atrial fibrillationsurgery when indicated may constitute a good option for the occasional young patient whose mitral valve is not amenable to repair and who is not contemplating pregnancy.
4.1 Previous related work
4.1.1 Survival, stroke, and reoperation
Other studies have also indicated thatin young patients, survival does not appear to be affected by the type of prosthesis [7,8]. A high incidence of thromboembolic and bleeding complications in patients with mechanical valves of nearly 3% per patient-year has previously been reported with mechanical prostheses[9]. However, the present series and previous work from our group indicate that thromboembolism rates are reduced in younger patients, as age is a risk factor [2]. Emery et al. reported that in patients under the age of 50 who hadAVR with a St. Jude Medical valve, freedom from mortality was 89% at 10 years and 87% at 18 years, with a freedom from thromboembolism of 96.5% at 10 years[10]. Other risk factors for death and thromboembolism, such as coronary artery disease, atrial fibrillation andpoor ventricular function, are less common in young patients [2,10].
Several mechanisms have been proposed to explain the process of early calcification of bioprostheses in young patients, including primary collagen degeneration, discrete immunologic reaction, increased turnover of calcium, and fatigue-induced lesions [11]. More recently, with the advent of the latest generatio nbioprostheses employing newer fixation and anti-mineralization processes [12], more promising long-term results ofbioprostheses in young patients have been published. Carpentier's group reported encouraging results with the high-temperature fixed valvular bioprostheses inyoung patients, illustrating 95% actuarial survival, 92% freedom from structural failure, 87.6% freedom from reoperation (91.7% for AVR, 80% for MVR) at 9 years. In a follow-up study of young adults aged 15–58 undergoing AVR with the Carpentier-Edwards supra-annular porcine bioprosthesis, Yamak et al. reported a freedom from SVD of 92% at 5 years and 44% at 12 years [13].
4.1.2 Quality of life
Young patients after valve replacement surgery face a full lifespan and need to lead an active lifestyle, including school or work attendance, regularphysical activity, and normal social relations, with the possibility of marriage and pregnancy. Although long-term anticoagulation does cause some degree of discomfort from blood tests and disruption of daily life [14], warfarin is well tolerated by most patients and does not have an important effect on quality of life [14,15]. Following mechanical MVR, van Doorn et al. reported thatyoung adults perceived their quality of life and level of functioning as normal or near normal in most areas compared with a reference population [14]. Similarly, good quality of life was reported by young patients several years after mechanical AVR [16,17]. Other authors have also found that the majority of young patients return to work after valve replacement, reporting an employment rate of 67–73% one year after surgery [18,19].
4.2 Limitations
4.2.1 Censoring and follow-up
The Cox proportional hazards regression method requires an assumption of independent censoring which may not always be met. In this regard, it is possible that patients lost to follow-up after a number of visits may have had subsequent outcomes that were not accounted for in the analyses.
4.2.2 Selection bias and generalizability
Like that of other observational cohorts, the results of these analyses may not be generalizable to all young adult patients who have undergone prosthetic valve replacement at other centers. Demographic and valve selection factors unique to the study cohort could have resulted in leverage from statistically influential patients. For example, the observation within mitral mechanical valves that tilting disc prostheses were associated with significantly more bleeding events than bileaflet prostheses despite a similar anticoagulation regimen may have reflected differences in referral patterns, patient compliance, or others. Confounding by indication, a form of selection bias that cannot be fully accounted for by multivariate analyses [20], might also have led to certain associations.
4.3 Conclusions
Despite the above limitations, the findings of this study allow patients and clinicians to better understand and quantify long-term survival, risks of adverse events, and quality of life after heart valve replacement in young adults. One important finding amongst others identified in this study is that the avoidance of smoking in young valve patients decreases thromboembolism and bioprosthesis reoperation hazards after valve replacement. As in the past, decisions on the choice of prosthesis need to bein dividualized and discussed between the patient and the treatment team, as no prosthesis is without major drawbacks in this agegroup.
Appendix. Conference discussion
Dr S. Cicek (Istanbul, Turkey): I saw from your patient population that you have very few patients younger than age over 30. Is that true?
Dr Mesana: Yes.
Dr Cicek: Then in your conclusion you say all these models may result in suboptimal long-term results. Do you consider changing your practice and using more Ross procedure for thesekinds of patients, or what is your comment on this?
Dr Mesana: We don't have many Ross procedures in this study. We have homografts thatwere used as a homograft reimplantation technique. I don't think that this question addresses the Ross operation. It is addressing that we offer suboptimal solutions for now with patients requiring AVR, and mechanical valves are probably not the best option even if there is a need for reoperation with a tissue valve.
Dr P. Kolh (Liege, Belgium): Can you elaborate a little bit on why the patients with mechanical valves are in worse physical condition? I assume it is not only because they have had thromboembolic events. There must be other reasons for that. Do you have any insights into that information?
Dr Mesana: Actually you have to understand that the number of strokes or bleeding complications are really low, in fact. But to elaborate on your question, we had the SF-12, the short form 12, which is a very simple questionnaire that we did through the phone, because not all patients were available, because Canada is a big country, and in Ontario we sometimes have a patient who lives a 20-h drive from our center, but we have almost a complete follow-up through the phone, and this is how they answered the question.
Dr Kolh: So it is a subjective assessment?
Dr Mesana: Yes.
Dr R. Dion (Leiden, Netherlands): What do you think is the reason why the mechanical valve would lead more often to a recurrence of congestive heart failure?
Dr Mesana: I cannot answer this question. This was just the result of our study. I think we have to go into more detail and try to find this out. It is a good question. I have no answer, to be honest.
Dr Dion: Do you think it isstill true that the adjunction of coronary bypass to a mitral valve or an aortic valve multiplies the risk of congestive heart failure by five?
Dr Mesana: I think it is well known data, but in our population that is how the univariate odds ratio showed up.
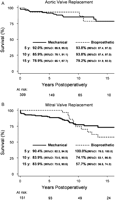
Late survival after aortic (A) or mitral (B) valve replacement in young adults. There was no significant survival difference between patients whounderwent aortic valve replacement only versus those who underwent mitral valve replacement, and no significant survival difference between patients implanted withmechanical versus bioprosthetic valves in any of the implant position. CI, confidence interval; y, years.
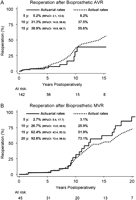
Actuarial and actual rates of reoperation after bioprosthetic aortic (A) or mitral (B) valve replacement in young adults. AVR, aortic valve replacement; CI, confidence interval; MVR, mitral valve replacement; y, years.
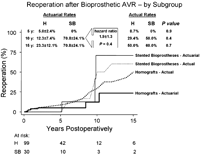
Actuarial and actual reoperation rates of aortic homografts (H) versus aortic stented bioprostheses* (SB) in young adults.Stented bioprostheses initially fared better than homografts but demonstrated a higher failure rate from 10 years onward, with no overall significant difference inreoperation rates between patients who received an aortic homograft and those who received a stented aortic bioprosthesis. H, aortic homograft patients; SB,stented aortic bioprosthesis patients; y, years. *Consisting of either a Medtronic Hancock or Edwards Perimount prosthesis in the series.
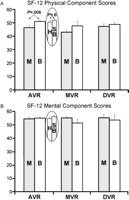
Physical (A) and mental (B) component scores of the short form (SF)-12 health status instrument. Physical component scores for AVR patients were significantly higher in patients with aortic bioprostheses than in those with aortic mechanical valves(P=0.006). This was observed regardless of whether aortic bioprosthesis patients received a homograft or a stented bioprosthesis, i.e. either a Medtronic Hancock or Edwards Perimount. There were no other significant differences in SF-12 physical or mental component scores between prosthesis types and subclasses. AVR, aortic valve replacement; B, bioprosthesis patients; DVR, double valve replacement; H, aortic homograft patients; M, mechanical valve patients; MVR, mitral valve replacement; SB, stented aortic bioprosthesis patients.
Presented at the joint 18th Annual Meeting of the European Association for Cardiothoracic Surgery and the 12th Annual Meeting of the European Society of Thoracic Surgeons, Leipzig, Germany, September 12-15, 2004.
We thank Linda Morrow for performing quality of life interviews and Mary Thomson for her continued assistance with the organization of the valve follow-upclinic.




