-
PDF
- Split View
-
Views
-
Cite
Cite
Alan D.L. Sihoe, Tak Wai Lee, Anil T. Ahuja, Anthony P.C. Yim, Should cervical ultrasonography be a routine staging investigation for lung cancer patients with impalpable cervical lymph nodes?, European Journal of Cardio-Thoracic Surgery, Volume 25, Issue 4, April 2004, Pages 486–491, https://doi.org/10.1016/j.ejcts.2003.12.026
Close - Share Icon Share
Abstract
Objectives: Detection of cervical N3 lymph nodes is currently not a routine preoperative investigation for lung cancer patients. We designed a study to assess if the frequency and accuracy of detection of metastatic cervical lymph nodes using cervical ultrasonography (US) and fine needle aspiration (FNA) justify their routine use in all lung cancer patients with impalpable cervical lymph nodes. Methods: Fifty patients with suspected and potentially operable non-small cell lung cancer were enrolled. Patients with palpable cervical lymph nodes were excluded. In addition to routine preoperative investigations, all patients received cervical US to determine the presence of cervical lymph nodes. Nodes suspicious of harboring malignancy according to a specific set of sonographic criteria (which include shape, echogenicity, nodal architecture, and vascular patterns) were subjected to biopsy by ultrasound-guided FNA. Results: Normal cervical lymph nodes were detected by cervical US in 30 patients (60%). Cervical lymph nodes suspicious of harboring malignancy were detected in 10 patients (20%). FNA confirmed cervical nodal metastasis in four of these patients (8%). The TNM staging of two patients (4%) was revised up to stage IIIb as a result, excluding them from further surgery. Cost analysis suggests this technique to be cost-effective when used as a routine preoperative investigation to exclude patients from unnecessary surgical intervention. No mortality or complications were encountered in all patients. Conclusions: Cervical US and FNA is a safe and cost-effective method of evaluating the status of impalpable cervical lymph nodes in lung cancer patients. Further study is warranted to establish the role of cervical US and FNA in lung cancer staging algorithms.
1 Introduction
Lung cancer is the leading cause of cancer death in the world [1], with the majority of patients having advanced inoperable disease at the time of presentation. Determining appropriate treatment requires accurate staging, of which lymph node assessment is an integral part. In modern lung cancer staging, emphasis is placed on the evaluation of the mediastinal lymph nodes. However, despite the advent of newer staging technologies such as positron emission tomography (PET), biopsy of these nodes is still often required for conclusive staging, involving invasive surgery such as mediastinoscopy or video-assisted thoracic surgery (VATS).
In comparison, the N3 station lymph nodes in the supraclavicular and scalene areas of the neck offer an alternative for staging without the need for such surgery. Up to 75% of lung cancer patients are estimated to harbor N3 station cervical lymph node metastases at the time of presentation [2]. Patients with N3 cervical lymph node metastases have stage IIIb disease, indicating cumulative survival rates at 1 and 5 years of only 32 and 3%, respectively [3]. Surgeons conventionally regard such patients as being inoperable, with surgery offering no survival benefit. On the other hand, prolonged survival may be possible if these patients are managed with aggressive chemo- and radio-therapy [4,5]. Identifying these patients can exclude them from unnecessary surgery, and direct them instead for aggressive oncological management. Should these cervical lymph nodes be palpable, percutaneous fine needle aspiration (FNA) can be easily performed [6]. However, in up to half of all cancer patients with cervical lymph node metastases, those nodes were impalpable at presentation [2,7], posing a diagnostic challenge to clinicians.
The ideal investigation for assessing cervical lymph node status has yet to be defined. Computed tomography (CT) is the mainstay of lung cancer staging, but its use in investigating lymph node status is limited by the lack of recognized size or morphological criteria by which a lymph node can be distinguished to be benign or malignant. Furthermore, most thoracic CT scanning protocols for lung cancer patients do not routinely include visualization of the supraclavicular and scalene lymph nodes. PET using 2-[18F]fluoro-2-deoxy-d-glucose can indicate the probability of malignant activity in a lymph node [8]. However, PET may fail to detect malignant lesions 4 mm or smaller in diameter, whereas unfortunately many metastatic cervical lymph nodes can be 5 mm or less in diameter [9–12]. Furthermore, PET has been reported to have relatively high false positive rates, with positive predictive values as low as 49% reported in the latest studies [13]. PET scanning is also a relatively expensive investigation, and is still unavailable in many centers.
Cervical ultrasonography (US), on the other hand, can detect small, impalpable metastatic neck lymph nodes in cancers of the head and neck with high sensitivity and specificity [10–12]. In one recent study, US proved superior to CT in detecting cervical lymph node metastases from lung cancer [14]. Cervical US is non-invasive, comparatively cheap, and readily available in most thoracic surgical centers around the world. Recent refinement of gray-scale and Doppler US technique has improved the radiologist's ability to identify cervical lymph nodes suspicious of harboring metastatic cells [15–17], targeting them for biopsy by FNA in the same sitting.
The idea of using cervical US to detect N3 lymph node metastasis in lung cancer patients is not new [9,18,19], but recent advances in cervical US technique and technology have prompted us to re-explore the role of modern cervical US in lung cancer staging.
2 Materials and methods
2.1 Patient selection and investigation
From August 1997 to November 1998, 78 consecutive adult patients with suspected primary non-small cell lung cancer referred to the Cardiothoracic Surgery Division of the Prince of Wales Hospital in Hong Kong were considered for enrollment to our study. The diagnosis of primary non-small cell lung cancer in each patient was suspected on the basis of initial sputum cytology, bronchoscopic biopsy, or percutaneous pulmonary FNA biopsy.
Patients were excluded from our study if they were subsequently found to have no malignancy (n=9), or if the lung malignancy was determined to have been metastatic from an extra-pulmonary source (n=4). Patients were also excluded if they had clinically palpable cervical lymph nodes at the time of presentation (n=8). Two patients refused participation in our study, and a further five patients had to be excluded when cervical US could not be arranged for them prior to lung resection surgery due to logistical issues. There remained 50 patients with primary non-small cell lung cancer with impalpable cervical lymph nodes who were then enrolled for this study. The study design was approved by the hospital Ethics Committee, and informed consent was obtained from all these patients.
The study group of 50 patients consisted of 37 men and 13 women. The mean patient age was 64.58 years (range 34–80 years). Seventeen patients had squamous cell carcinomas, 16 adenocarcinomas, 13 poorly differentiated non-small cell carcinomas, two large cell carcinomas, one mucoepidermoid carcinoma, and one lymphoepithelioma-like carcinoma.
Routine pretreatment investigations for all 50 patients included a chest radiograph, fibre-optic bronchoscopy, chest CT with intravenous contrast, abdominal US, and technetium (Tc-99m) pyrophosphate bone scanning. Preliminary staging was assigned using the 1997 update of the TNM international staging system [3]. On the basis of these investigations (excluding cervical US), five patients were initially determined to be in stage Ia, 19 in Ib, three in IIb, 10 in IIIa, eight in IIIb, and five in IV.
2.2 Cervical ultrasonography and fine needle aspiration
All 50 patients underwent cervical US by one experienced radiologist (ATA). US scanning was performed using an ATL HDI 5000 ultrasound scanner (Phillips Ultrasound, USA) with a 7–12 MHz real time linear-array transducer. Examination was performed using a continuous transverse and sagittal sweep technique, covering the entire neck on both sides from the thoracic outlet and scalenous muscles up to the submental and retroparotid regions. The N3 station cervical lymph nodes were defined as those scalene and supraclavicular nodes illustrated by the TNM system of 1997 [3]. Based on the US findings, each patient was classified as having no detectable cervical lymph nodes, normal or benign lymph nodes, or lymph nodes sonographically suspicious of metastases.
Specific grey-scale and power Doppler sonographic criteria were employed to distinguish normal benign cervical lymph nodes from those suspicious of being malignant. These included shape (short axis to long axis ratio, S/L), echogenicity of the hilus, node echogenicity, internal architecture, vascular pattern, and intranodal vascular resistance. Benign nodes tend to be elliptical (S/L<0.5), hyperechoic and have an echogenic hilus [16,20] (Fig. 1) . Benign nodes show hilar vascularity with power Doppler US, and have a low intranodal vascular resistance with spectral Doppler US (resistive index, RI<0.7, 70%; pulsatility index, PI<1.5, 94%) [17] (Fig. 2) . Metastatic nodes are predominantly round in shape (S/L>0.5), hypoechoic and without echogenic hilus [16,17] (Fig. 3) . Intranodal cystic necrosis is common in metastatic nodes from squamous cell carcinomas [20]. With power Doppler US, metastatic cervical lymph nodes tend to show peripheral and mixed (both hilar and peripheral) vascularity, and have a high vascular resistance with spectral Doppler US (RI>0.7, 86%; PI>1.5, 74%) [17] (Fig. 4) .
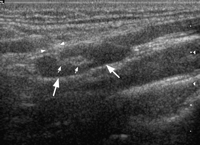
Gray-scale sonogram showing a longitudinal scan of a normal upper cervical node which is hypoechoic and elliptical in shape (large arrows). The lymph node demonstrates an echogenic hilus (small arrows), which is continuous with the adjacent soft tissues (arrowheads).
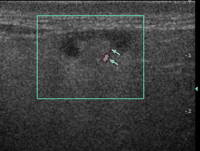
Power Doppler sonogram showing a normal parotid lymph node with hilar vascularity (arrows).
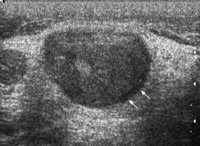
Gray-scale sonogram showing a transverse scan of a metastatic supraclavicular node (arrows). The lymph node is hypoechoic, well defined, round shaped and without echogenic hilus.
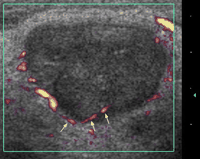
Power Doppler sonogram of the same lymph node in Fig. 3. Note that the lymph node shows peripheral vascularity, which is along the periphery of the node and enters the node (arrows).
Any lymph node suspicious of harboring metastases was biopsied with percutaneous FNA in the same sitting using a sterile technique and local anesthesia. At least two samples of each suspicious node were obtained using a 22-gauge needle and syringe. The smears of the FNA aspirates were sent for cytological analysis by two experienced cytologists.
3 Results
3.1 Cervical ultrasonography and fine needle aspiration
Of the 50 patients in the study group, 10 patients (20%) had no sonographically detectable cervical lymph nodes. Normal or benign cervical nodes according to our above criteria were found in 30 patients (60%). Cervical nodes judged to be suspicious of metastases were found in 10 patients (20%).
The 10 patients with suspicious cervical lymph nodes underwent FNA biopsy of the nodes under US guidance. The results of the FNA biopsies are summarized in Table 1 . In four of these 10 patients (8% of total), malignant cells were confirmed in the suspicious cervical lymph nodes, confirming them to have N3 nodal metastasis. In two patients, FNA yielded insufficient material for a definite cytological diagnosis. However, in both these patients, there was evidence of distant metastatic spread (M1 disease) in other subsequent investigations (liver metastasis in one, bilateral pulmonary metastases in the other). Of the four patients with primarily negative FNA results, two had subsequent CT evidence of extensive local tumor infiltration (T4 disease) precluding surgery. These patients were not subjected to another FNA as the result would therefore not have altered their overall management.
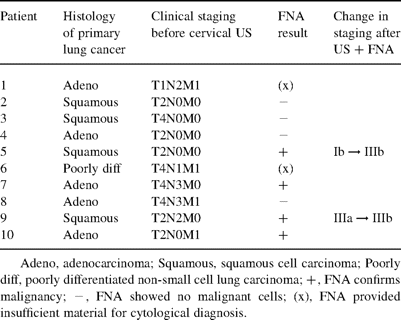
Ten patients in whom cervical ultrasonography demonstrated cervical lymph nodes suspicious of metastasis
No complications, mortality or significant morbidity related to the cervical US or FNA was encountered in all 50 patients in this study.
3.2 Clinical correlation
The initial staging of the patients after routine pretreatment investigations was correlated with the findings on cervical US. The results are summarized in Table 2 . We consider lung cancer at stages I and II to be potentially operable, and at IV to be categorically inoperable. For this study, we consider patients with stage IIIb disease to be inoperable, although we are aware of reports of satisfactory surgical outcomes in these patients after induction chemotherapy or combined chemo- and radio-therapy [5]. For stage IIIa disease, we would consider surgery only in selected patients. For stage IIIa patients with suspicious N2 lymph nodes, we routinely offer mediastinoscopy and only proceed to curative lung resection if metastases in these nodes were excluded. Based on these criteria, 26 patients (52%) were recognized as having operable early stages of disease, 15 patients (30%) as having inoperable advanced stage, and 9 patients (13%) as having stage IIIa disease.

Correlation of cervical ultrasonography findings with results of pretreatment staging investigations
Cervical US with FNA confirmed N3 metastatic lymph nodes in four patients (8% of the total). These four patients were initially classified as having stage Ib (T2N0M0, squamous), stage IIIa (T2N2M0, squamous), stage IIIb (T4N3M0, adenocarcinoma), and stage IV (T2N2M1, adenocarcinoma) disease, respectively. In two patients, the cervical US and FNA result changed them from having a potentially operable stage of disease (stage Ib and IIIa) to having an inoperable stage (stage IIIb). In other words, two patients (4% of the total) were excluded from further invasive surgical procedures as a result of the cervical US and FNA.
We attempted to identify any patient parameters that may be associated with the presence of N3 cervical nodal metastasis as confirmed by FNA. All statistical operations were performed using the Mann–Whitney test for numeric variables or the Fisher exact test for non-numeric variables. A P-value of less than 0.05 was regarded as indicating significance in the association between any parameters. Using these statistical methods, we were unable to detect any significant association between the presence of N3 cervical lymph node metastases and any demographic, clinical, histological or radiological parameter for our study group of 50 patients.
4 Discussion
In this study, we have shown that by using simple cervical US, a modern set of specific sonographic criteria for assessing the nodes, and US-guided FNA biopsy, we can identify 8% of lung cancer patients as having N3 lymph node metastases. Using this technique, 4% of lung cancer patients had their clinical staging upgraded from a potentially operable stage to an inoperable stage IIIb.
Although the concept of open biopsy of cervical lymph nodes for lung cancer staging has been advocated since 1949 [21], we are aware of only a handful of other studies previously describing US-guided cervical lymph node biopsy [9,14,18,19]. Two studies reported the routine use of cervical US in a similar fashion to our study, but used only size to indicate suspicious nodes, as opposed to the more sophisticated criteria in our study [9,14]. Table 3 illustrates the comparison between our study and these latter two studies.
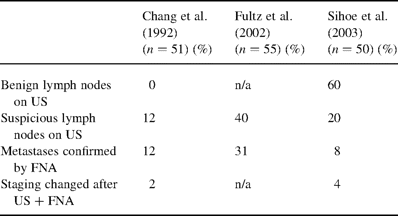
Comparison of studies employing cervical US to detect impalpable cervical lymph nodes in lung cancer patients
In 1992, Chang and colleagues detected cervical lymph nodes in six (12%) out of 51 lung cancer patients using cervical US [9]. Metastasis in the N3 neck lymph nodes was confirmed by FNA in all six patients. In comparison, we have detected cervical lymph nodes (both benign and malignant) in 80% of our patients, probably reflecting the enhanced sensitivity of more modern cervical US technology. The rates of N3 metastases found (8 versus 12%) are comparable, perhaps demonstrating that our US criteria to define suspicious lymph nodes are sufficiently specific and discriminating despite the many benign nodes detected.
In 2002, Fultz and associates described a combination of cervicothoracic CT with cervical US to detect cervical lymph node metastases in lung cancer patients [14]. They found such metastases in 17 out of 55 patients (31%) using this approach. However, US alone was shown to be more sensitive than CT in detecting metastatic cervical lymph nodes (CT failed to detect 18% of the metastatic nodes found by US). Compared to our results, Fultz's study found greater numbers of both suspicious lymph nodes, and FNA-confirmed N3 nodal metastases. This difference may be due to a patient selection bias. Patients in Fultz's study appear to be recruited from a variety of both in-patient thoracic surgery and pulmonary medicine services, and out-patient referrals from primary care physicians [14]. In contrast, all the patients in our study were referred from specialist hospital internists and oncologists to a specialist cardiothoracic surgery unit specifically for consideration of curative surgery. Whereas around 20% of all lung cancer patients have been estimated to ultimately undergo surgery [22], in Fultz's study this figure was 24%, and in our study it was over 50%. This suggests that patients in our study may in effect have been screened by their referring specialist physicians to be potentially operable, and may therefore constitute a group biased towards having earlier-stage, operable disease.
At present, ours is a feasibility study aiming to re-define a role for cervical US in lung cancer patients. As such, it does have limitations. Our numbers are as yet inadequate to demonstrate statistically significant associations between the US and FNA findings with any other clinical, histological or demographic parameter. We are also aware that our technique may potentially still miss focal or microscopic lymph node involvement. We have not defined the sensitivity and specificity of this technique. Instead, we have relied on previously established data proving that cervical US has very high sensitivity and specificity for lymph node staging of various head, neck and esophageal malignancies [14–17,20]. We are planning further studies to correlate cervical US accuracy with postmortem findings in lung cancer patients.
Nonetheless, our results have shown that routine cervical US for lung cancer patients is safe, feasible and well tolerated by patients. We incurred no complications, mortality or morbidity in this study. No participating patient refused the US or FNA after recruitment to the study.
We can put our results into context by comparing them with other established investigations for extra-thoracic metastases. In otherwise operable lung cancer patients, it has been estimated that 3.3% of patients were spared from unnecessary thoracotomy by CT of the head, 4.7% by CT of the adrenals, 9.3% by radionucleotide bone scanning, and 2.3% by radionucleotide and US liver imaging [23]. Against these figures, our rate of 4% using cervical US is comparable. In addition, it should be noted that US is simpler to arrange and cheaper to perform than most of these other investigations.
However, the latest guidelines suggest that the above investigations for extra-thoracic metastasis should not be routinely performed in the absence of indicative clinical symptoms or signs [24]. A significant part of the argument is that they are not cost-effective, given their relatively low rates of positive yield. To justify the routine use of our technique, we therefore considered its cost-effectiveness. Each cervical US plus an US-guided FNA of a neck LN costs approximately US$100 to perform in our institution. As we have demonstrated that 4% of patients could potentially be spared from fruitless surgical intervention by this technique, it would take 25 cervical US and FNA procedures, costing approximately US$2500 ($100 × 25 patients), to exclude one patient from further surgery. In contrast, it has been estimated that the mean hospital charge for a single patient undergoing surgery with curative intent for lung cancer is in excess of US$11 000 [25]. On this basis, the technique of cervical US and FNA appears to be cost-effective. Therefore, in contrast to other investigations for extra-thoracic metastases, there may be an argument in favor of incorporating our technique in routine lung cancer staging algorithms.
Appendix A Conference discussion
Dr W.S. Walker (Edinburgh, Scotland): When you do your cost-effectiveness, it's in comparison with not doing it, not with using any alternate method of assessing nodes; is that correct?
Dr Sihoe: That's true, but what we're actually doing, we're just adding this investigation on top of what we normally do for staging for all lung cancer patients. So any benefit from this study is purely in addition. It's a benefit, and it seems apparently that there's very little cost at the moment.
Dr P. De Leyn (Leuven, Belgium): What was the staging of the disease? They were potentially operable patients, but did they have a mediastinoscopy? What was the mediastinal staging of these patients, what was the proportion of patients with N2 disease?
Dr Sihoe: To answer that, our cohort of patients was geared towards early staging. These patients were referred to us from primary care physicians and respiratory physicians who had already interviewed these patients, examined them physically, and they thought they were operable. So by the time they referred them to us and we continued the routine operative staging, at the end we found that over 50% of our patients were operable stage I and II. According to my memory, I think there were about 10 patients in stage IIIA.
Dr De Leyn: How many of these patients had enlarged nodes on CT scan? I just want to know something about the initial staging of these 50 patients.
Dr Sihoe: I think that highlights one of the problems of modern CT in that most thoracic CT protocols now do not routinely include cuts of the neck, and that is certainly the case in our hospital. They don't actually go very high up. Most protocols just go up to the apex of the lungs. So I don't think that in any of our patients we actually looked for enlarged lymph nodes in the neck on the CT.
Dr H. Wertzel (Lostau, Germany): If you find a lymph node on sonography, are you always sure that you get by puncture the correct histology or cytology?
Dr Sihoe: At the moment, in our study, a very simple study, we have not yet defined the sensitivity and specificity of the ultrasound-guided FNA. What we are relying on as a basis are studies done in head and neck cancers. We are using a similar technique to those which have claimed accuracies of over 80%. In fact, some studies have claimed sensitivities of up to 90%, I think the highest one I've seen is 93%, for ultrasound-guided FNA. This is compared to actual autopsies and open biopsies that we've done. But you are right, we can't claim a sensitivity as yet from this study.




