-
PDF
- Split View
-
Views
-
Cite
Cite
Xin Wang, Minxin Wei, Pekka Kuukasjärvi, Jari Laurikka, Otso Järvinen, Timo Rinne, Eva-Liisa Honkonen, Matti Tarkka, Novel pharmacological preconditioning with diazoxide attenuates myocardial stunning in coronary artery bypass grafting, European Journal of Cardio-Thoracic Surgery, Volume 24, Issue 6, December 2003, Pages 967–973, https://doi.org/10.1016/S1010-7940(03)00438-X
Close - Share Icon Share
Abstract
Objective: To investigate whether novel pharmacological preconditioning with diazoxide could protect the myocardial function and decrease myocardial injury in patients undergoing coronary artery bypass grafting (CABG). Methods: Forty patients with stable angina who were scheduled for isolated elective CABG operations were randomized into control group (n=20) and diazoxide (DZX) group (n=20). In the DZX group, 1.5 mg/kg diazoxide was infused intravenously within 5 min followed by a 5-min washout before commencing the cardiopulmonary bypass (CPB). In the control group, a time-matched period of placebo infusion was given. Hemodynamic data and biochemical markers of myocardial injury were measured perioperatively. Results: There were no adverse effects related to diazoxide. Cardiac index (CI) increased postoperatively as compared with baseline. In the DZX group, the improvement of CI was better than that in the control group (p=0.001). Left and right ventricular stroke work indexes decreased postoperatively, and recovered much faster in the DZX group (p=0.027 and p=0.049, respectively). There were no statistically significant differences in the other hemodynamic parameters. The creatine kinase cardiac isoenzyme (CK-MB) was highest in both groups on the first postoperative day (control 28.8±23.8 and DZX 27.3±19.4, N.S.). The cumulative release of CK-MB postoperatively was lower in the DZX patients as compared with the controls, but the difference remained not significant (p=0.09). Conclusions: Pharmacological preconditioning of the human heart with diazoxide is feasible; it confers additional myocardial protection beyond that provided by the cardioplegia alone by attenuating myocardial stunning after CABG operations.
1 Introduction
The optimal myocardial protection against ischemic injury during cardiac surgery still remains to be pursued. Ischemic preconditioning is one alternative among effective cardioprotective strategies. However, majority of the cardiac surgeons are reluctant to accept single or repeated clamping of the aorta required for mechanical ischemic preconditioning because of the risk of atheroemboli. Thus, extensive research has been aimed at identifying pharmacological agents to mimic ischemic preconditioning in hope of achieving clinical benefit.
A huge body of research over more than a decade has focused on ischemic preconditioning in virtually every species, and some of the underlying signaling pathways have been resolved. It is well known that G-protein coupled receptors are activated by some agonists, such as adenosine [1], bradykinin [2] and opioids [3]. Receptor activation triggers a signaling pathway and leads to activation of several kinases, in particular protein kinase C (PKC) [4], and the subsequent opening of mitochondrial adenosine triphosphate sensitive potassium (mitoKATP) channels [5,6]. Although the controversy exists as to whether mitoKATP channel is a mediator or the end-effector, there is a consensus that mitoKATP channel plays a major role in ischemic preconditioning.
Diazoxide is a selective mitoKATP channel opener [6] conferring cardioprotection in animal models [5,7–9] of myocardial infarction and in vitro cultured human cardiomyocytes [10] subjected to ischemia–reperfusion stimulus. Administering diazoxide prior to the onset of ischemia could produce a significant reduction of myocardial infarct size in anaesthetized rat [7,11] and rabbit [8]. It also improves the myocardial contractile function in Langendorff rat model [5] and tissue viability in human cardiomyocytes [10]. To date, there is no evidence that such a cardioprotection elicited by diazoxide is involved in the intact human heart. Thus, the present study sought to investigate whether pharmacological preconditioning with diazoxide might be able to protect the heart function and limit myocardial injury in patients undergoing coronary artery bypass grafting (CABG) operation.
2 Materials and methods
2.1 Patients
The study was approved by the Ethics Committee of Tampere University Hospital, and written informed consent was obtained from each patient.
Forty patients with stable angina were scheduled for isolated elective CABG operations and were randomized into the control group and diazoxide (DZX) group. Patients were excluded for any of the following: (1) diabetes mellitus treated with sulfonylurea drugs, (2) unstable angina, (3) recent myocardial infarction (<1 month) (4) prior CABG surgery, (5) with heptic, renal or pulmonary disease.
2.2 Study protocol
DZX group patients received diazoxide (Hypertonalum, ESSEX Pharma German) infusion prior to the initiation of cardiopulmonary bypass (CPB). The dose of diazoxide (1.5 mg/kg) was diluted in 20 ml of 0.9% sodium chloride. This was administered via central venous port of a Swan–Ganz catheter as a 5-min period, followed by 5-min washout period. Thereafter CPB was started. Control patients underwent a time-matched (10 min) period of placebo (0.9% sodium chloride) infusion. To avoid hypotension during the infusion of the study medicines, central venous and pulmonary diastolic pressure were maintained at a level not less than the baseline values obtained before the induction of anesthesia. Whether the systolic blood pressure decreased more than 20% compared with pre-infusion value, or the absolute value decreased below 70 mmHg, CPB was commenced immediately to avoid hemodynamic deterioration. Should this happen, the study infusion was completed during the CPB.
2.3 Anesthesia and surgical methodology
A radial artery line and a pulmonary artery catheter were inserted for hemodynamic monitoring. Anesthesia was induced with propofol (0.5–1.0 mg/kg), sufentanil (0.8–1.0 μg/kg) and rocuronium. Propofol infusion was continued with a rate of 50–80 mg/kg/min and sufentanil with 0.03–0.05 μg/kg/min. Additional midazolam boluses were given as necessary. Halogenated anesthetic gases were restricted completely due to possible influence on the KATP-channels. Occasional hypertension was controlled with nitroglycerine or labetalol.
The surgical techniques were standardized in all cases. A median sternotomy was performed. CPB was established with regular cannulation technique using mild hypothermia (34 °C). A retrograde coronary sinus cannula was inserted transatrially for cardioplegia infusions and study sampling. The first cardioplegia infusion was given antegradely for 2 min, and then retrograde cardioplegia were given for another 2 min. Subsequent 1-min retrograde cardioplegia infusion was administered after each distal anastomosis, and final warm retrograde cardioplegia (37 °C) was given for 3 min before aortic declamping. The proximal anastomoses were constructed during a single cross-clamping period. After weaning the CPB, inotropes (noradrenaline and dopexamine) were used to maintain hemodynamic performance if the cardiac index (CI) was less than 2.0 L/min/m2. They were used at time points when hemodynamic data were recorded and continued for at least 6 h.
2.4 Measurement and data acquisition
2.4.1 Biological markers
2.4.1.1 Creatine kinase cardiac isoenzyme and troponin I
Blood levels of creatine kinase cardiac isoenzyme (CK-MB) and troponin I (TnI), two biochemical markers of cellular necrosis, were serially measured. Blood samples were taken from peripheral vessels after the induction of anesthesia (baseline), at 6 h, at 12 h after CPB, and on the first and second days after surgery. All samples were collected and cooled at 4 °C immediately, then centrifuged. Serum sample were measured with a Chiron ACS180 analyzer. (ACS: 180; Chiron/Diagnostics, Emeryville, CA) using a direct chemiluminescence method.
2.4.1.2 Lactate
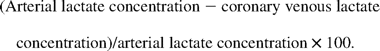
2.4.2 Hemodynamic data
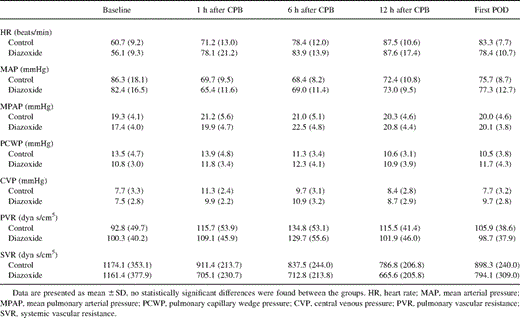
Hemodynamic data were serially collected at the following five time points: (1) before the induction of anesthesia as the baseline, (2) 1 h after CPB, (3) 6 h after CPB, (4) 12 h after CPB, and (5) on the first postoperative morning. Heart rate (HR), mean artery pressure (MAP), mean pulmonary artery pressure (MPAP), pulmonary capillary wedge pressure (PCWP), central venous pressure (CVP), and cardiac output (CO) were measured. Derived cardiovascular variables, including CI, left ventricular stroke work index (LVSWI), right ventricular stroke work index (RVSWI), systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) were calculated using standard formulas.
2.5 Statistics
Statistical analysis was performed using SPSS (SPSS for Windows, Version 10.1; Chicago, IL) software package. With a minimum sample size of 18 cases per group the study could have achieved 90% power at alpha error level 0.05 to detected change of cardiac index at least 0.8 L/min/m2. The student's t test or Mann–Whitney U test was used to distinguish differences in demographic parameters between the groups. Continuous variables were analyzed by analysis of variance for repeated measures. Logarithmic transformation was used when the variables were not normally distributed. The preoperative values were taken as covariates, and changes with respect to preoperative values were assessed. Statistical significance was attributed to p values lower than 0.05. All results are expressed as mean±standard deviation (SD).
3 Results
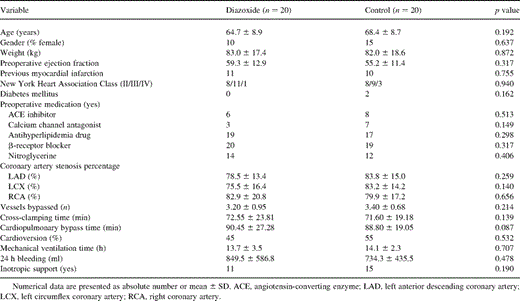
All the 40 patients fulfilled the study protocol. The patients' demographic data and perioperative data are presented in Table 1 . Preoperative patient characteristics for the two groups were similar. There were no significant inter-group differences with regard to age, sex, New York Heart Association class, ejection fraction, diseased vessel, preoperative medication, number of grafted vessels, mechanical ventilation time and inotropic support. Twenty patients who were treated with diazoxide suffered a mild hypotension with a decrease in systolic blood pressure 14.8±4.5% compared with pre-infusion value. Earlier than planned CPB was not needed in any of the study patient. There were no adverse effects related to diazoxide. The diazoxide group patients had slightly longer CPB and cross-clamping times, but the differences to the controls were not statistically significant (p=0.087 and p=0.139, respectively). There was no major complication in either group. All patients survived the operation and were discharged from the hospital.
3.1 Hemodynamic data
CI increased over time compared with baseline in both groups except 1 h after CPB in the control group (Fig. 1 ). In the DZX group, the increase of CI was greater than that in the control group (p=0.001). LVSWI decreased in both groups (Fig. 2 ), but it recovered much faster in the DZX group (p=0.027). Also RVSWI decreased 1 h after CPB in the controls, whereas in the DZX group it increased (Fig. 3 ), and this change was statistically significant between the two groups (p=0.049). There were no statistically significant differences in HR, MAP, MPAP, PCWP, CVP, SVR and PVR between the groups (Table 2 ).
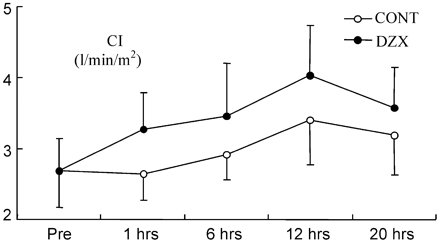
CI prior to the CABG (pre) and at time points after declamping of the aorta. Values are given as mean±SD. (p=0.001, analysis of variance with repeated measures).
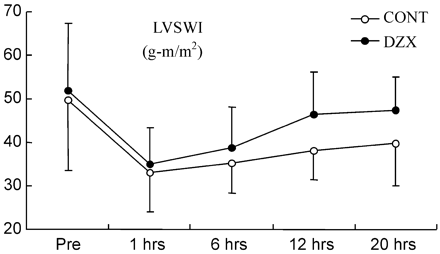
The LVSWI prior to the CABG (pre) and at time points after declamping of the aorta. Values are given as mean±SD. LVSWI, left ventricular stroke work index. (p=0.027, analysis of variance with repeated measures).
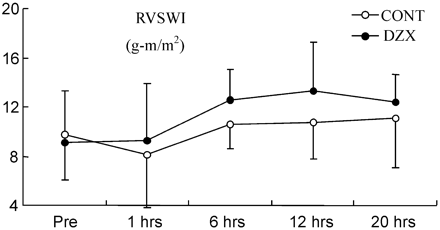
The RVSWI prior to the CABG (pre) and at time points after declamping of the aorta. Values are given as mean±SD. RVSWI, right ventricular stroke work index. (p=0.049, analysis of variance with repeated measures).
3.2 Biochemical markers
The baseline levels of CK-MB (5.45±5.68 and 5.43±2.52 in the DZX and control groups, respectively, p=0.99) and of TnI (0.20±0.01 and 0.23±0.08 in the DZX and control groups, respectively, p=0.21) did not differ. Both TnI and CK-MB reached peak value on the first postoperative day. The peak values of CK-MB and TnI were 27.3±19.4 and 6.9±2.1, respectively, in the DZX group, whereas in the control group they were 28.8±23.8 and 8.1±2.3, respectively. Diazoxide patients released less CK-MB than controls postoperatively, but the difference was not significant (Fig. 4A , p=0.09). The release of TnI was similar in both groups (Fig. 4B, p=0.54). The lactate extraction ratio was closely the same between the groups (p=0.81).
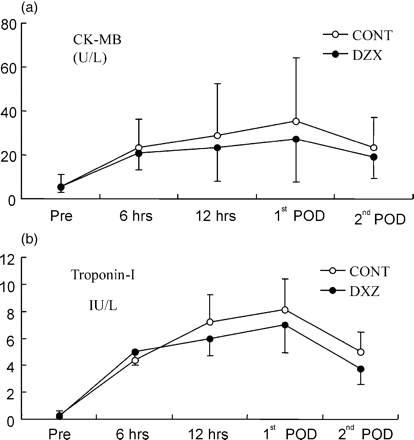
CK-MB (A) and CTnI (B) prior to the CABG (pre) and at time points after declamping of the aorta. Values are given as mean±SD. (p=0.09, analysis of variance with repeated measures).
4 Discussion
The results of the present study demonstrate that a novel pharmacological preconditioning with diazoxide (1.5 mg/kg) prior to commencing CPB appears to confer additional myocardial protection beyond that provided by the cardioplegia alone, as manifested by a better hemodynamic recovery and tendency to less release of CK-MB postoperatively.
To date there is a large body of evidence derived from animal and in vitro human tissue models [8,10,12] that diazoxide, as a selective mitoKATP channel opener [6], can mimic ischemic preconditioning and improve cardiac contract function and cellular viability. Previous studies using rat [5], rabbit [8,12], dog [9] and isolated human myocardial tissue [10] have shown that diazoxide can elicit protective effects of ischemic preconditioning which is manifested as a reduced infarct size, reduced release of CK-MB and improved contractile function. This protection has been abolished by using selective mitoKATP channel blockers. However, the mechanism of protection by opening mitoKATP channels remains unknown. All these inspiring results of diazoxide-induced preconditioning have been associated with an expectation that pharmacological preconditioning could become available for practical human use.
It is well established that myocardial stunning plays a central role in the postoperative heart dysfunction. Stunned myocardium was initially described by Heyndrickx et al. as “prolonged postischemic dysfunction of viable tissue salvaged by reperfusion” [13]. Stunned myocardium is characterized by normal blood flow and diminished contractile efficiency. Several clinical reports have demonstrated depressed ventricular function in the initial hours after CABG operations, and found that this dysfunction is usually resolved within 24–48 h, and that it does not appear to be dependent on alterations in preload and afterload [14]. Our results were well in concordance with these findings. We also noted that in the control group, CI, LVSWI and RVSWI decreased postoperatively and reached a nadir 1 h after CPB, CI and RVSWI recovered within 24 h, and also LVSWI later improved. Compared with the controls, the heart functional impairment was significantly less in the diazoxide group, as indicated with better CI and faster recovery of RVSWI and LVSWI within 24 h. Other indicators of the preload and afterload of left and right ventricles, with measurements of PCWP, SVR and CVP, PVR, respectively, were almost similar between the groups. Therefore the hemodynamic improvement brought by diazoxide was mostly from less stunned myocardial function and was manifested as a better recovery of contractility.
Since another primary effect of diazoxide preconditioning in animal experiments has been a reduction of infarct size, we measured cellular injury with the release of CK-MB and TnI that are two sensitive biochemical markers of cellular necrosis. Our results demonstrated that the release of CK-MB and TnI was slightly lower in the diazoxide group than in the control group, a finding that is consistent with the beneficial cardioprotective effects of diazoxide on hemodynamic parameters. The difference between the groups failed to achieve statistical significance probably because of the small sample size and the fact that our patients were selected from a low-risk population, where the good distribution of cardioplegia had already conferred an effective limitation on ischemic damage, and makes it more difficult to demonstrate further significant improvements.
There are some issues that could complicate the assessment of the efficacy of diazoxide preconditioning. Many preoperative medications could bias the results. Firstly, some oral hypoglycemic agents are inhibitors of the KATP channel. Garratt et al. demonstrated that patients who receive sulfonylurea drugs have been jeopardized at an increased relative risk of cardiovascular mortality after direct angioplasty for myocardial infarction [15]. It is suggested that sulfonylurea drugs will elicit the resistance to protection by KATP channel opener. Recognizing this negative effect, we excluded any patients who had received hypoglycemic treatment. Secondly, preoperative administration of opioids [16] and halothane anesthetic gas [17,18] has been noted to precondition heart in both laboratory and clinical settings. Isoflurane, a routine anesthetic gas, has been proved to elicit a beneficial effect on heart function and to limit necrosis in CABG patients [18]. Given such caveats, the study protocol was designed to minimize any possible confounding factors by removing isoflurane, so that the control group may not gain extraneous beneficial treatment that could obscure the effect of diazoxide preconditioning in the study group. Thirdly, there is a limitation to use the hemodynamic parameters such as CI, LVSWI and RVSWI as the end points to evaluate the benefit derived from the preconditioning. These clinical end points could be influenced by patients' status of pre- and afterload and many therapeutic interventions. Fourthly, the inotropic support may also mask the hemodynamic data. Stunned myocardium typically is able to contract when exposed to inotropic stimuli [19]. Since in our institution the postoperative control of blood pressure by using inotropes was common, a high percentage of patients received inotropic support (mainly noradrenaline to control blood pressure). Although there was no statistical significance in the difference between the groups, this effect could more or less bias the results. Finally, a minimum duration of ischemia is required to activate endogenous preconditioning response [20,21], and the duration of ischemia required to elicit preconditioning is known to vary between species [22]. A certain threshold of stimulation has to be reached. Our study showed that 1.5 mg/kg diazoxide infusion during 5 min prior to CPB did not elicit severe or prolonged hypotension or hemodynamic instable (about 10–15% systolic blood pressure decreased). The present results demonstrated that such a dosage protocol might reach the preconditioning threshold, and augment the heart function. Previous studies [5,23] have indicated that high concentrations of mitoKATP channel opener might have greater efficacy in protecting myocardium. The present study was not designed to address this problem, and further investigations are needed to find out as optimal clinical protocol.
In summary, the present study indicates that pharmacological opening of mitoKATP channels with diazoxide (1.5 mg/kg) prior to commencing CPB in CABG patients with stable angina resulted in significant improvement in hemodynamic performance as well as in tendency of reducing postoperative CK-MB release as compared with the cardioplegia alone. Attenuated myocardial stunning was a benefit of this additional protection. The data also suggested that there is still some room left for pharmacological additives, such as diazoxide, to confer preconditioning in the human heart. The exact underlying mechanism and optimal dose of diazoxide-induced precondition in clinical setting warrants further study.
The present study was supported by grants from the Medical Research Fund of Tampere University Hospital and the CIMO foundation.
References
- myocardium
- creatine kinase
- angina, stable
- coronary artery bypass surgery
- cardiopulmonary bypass
- hemodynamics
- myocardial stunning
- hemodynamic measurements
- cerebrovascular accident
- ischemic stroke
- heart arrest, induced
- biochemical markers
- heart ventricle
- isoenzymes
- heart
- pharmacology
- diazoxide
- cardiac index
- myocardial injury
- infusion procedures
- doppler hemodynamics
- creatine kinase mb isoenzyme




