-
PDF
- Split View
-
Views
-
Cite
Cite
Kenji Hazama, Akinori Akashi, Norihisa Shigemura, Tomoyuki Nakagiri, Less invasive needle thoracoscopic laser ablation of small bullae for primary spontaneous pneumothorax, European Journal of Cardio-Thoracic Surgery, Volume 24, Issue 1, July 2003, Pages 139–144, https://doi.org/10.1016/S1010-7940(03)00181-7
Close - Share Icon Share
Abstract
Objective: The purpose of this study was to establish a new surgical technique of thoracoscopic laser ablation for the patients of primary spontaneous pneumothorax (PSP) with small bullae, by using endoscopic equipment with a 2-mm diameter. Method: According to the size of a bulla identified by high-resolution computed tomography (HRCT), we have a protocol to determine an indication; the conventional video-assisted thoracic surgery (VATS) procedure by both stapler bullectomy and laser ablation to visceral pleura surrounding the bulla (bullae size: greater than 2 cm), or a new VATS procedure using needle shaped thoracoscopy and endoscopic equipment with a 2-mm diameter (needle VATS) by laser bulla ablation alone (bullae size: less than 2 cm). Results: The conventional VATS was performed in 54 patients and needle VATS in 60 patients. In the needle VATS group, operation time was shorter than that of the VATS group (39±17 min vs. 56±22 min). Use of non-steroidal anti-inflammatory drugs for postoperative wound pain could be reduced in the needle VATS group (3% vs. 56%). There were no complications in the needle VATS group, but three complications (5.6%) in the VATS group, including prolonged air leakage (>4 days) in two and refractory intercostal pain in one. The rate of recurrence after the operation was similar in both groups (3.7% vs. 3.3%). The needle VATS allowed wound healing without a scar and reduced the patient's cosmetic problems. Conclusion: The needle VATS procedure for patients with a bulla size less than 2-cm diameter was as useful as the conventional VATS procedure.
1 Introduction
Video-assisted thoracic surgery (VATS) for patients with primary spontaneous pneumothorax (PSP) has become a popular procedure because it is less invasive than conventional thoracotomy [1–6]. VATS procedure has usually been carried out using both a thoracoscope and endoscopic stapling devices of 10 mm in diameter through surgical ports for bullectomy [1–3,6,7]. Advances in endoscopic surgery are facilitating the development of miniaturized scopes and related instruments. We have tried to establish a new surgical technique of thoracoscopic laser ablation for the patients of PSP with small bullae by using endoscopic equipment with 2-mm diameter. In this article, we report our experience and the results for our new endoscopic procedure, termed needle VATS, that provides patients with less invasiveness and more cosmetic benefit.
2 Patients and methods
Between January 1998 and December 2000, we treated 114 consecutive patients with PSP by endoscopic surgery in our institution. The surgical indications were 91 patients (80%) with ipsilateral recurrence of PSP, 19 patients (17%) with persistent air leakage, and four patients (3%) with contralateral recurrence. Eighty-five patients were male, and 25 were female. The mean age of patients was 22.5 years, ranging from 14 to 70.
Patients with PSP were admitted to our institution. A chest tube of 2 mm in diameter (Argyle, USA) was inserted into the fourth intercostal space at the midaxillary line in all patients. After identification of re-expansion of the lung, high-resolution computed tomography (HRCT) of the chest was examined. All CT scans were obtained with an X-Vigor scanner (Toshiba Medical, Tokyo, Japan), with a scanning time of 1 s. Full-inspiration thin-section CT was performed by using 2-mm collimation every 2 mm from the lung apex to the superior segment of the lower lobe. The number, size, and location of the bullae were evaluated. We considered surgical treatment if a bulla was visible and a patient agreed to it. According to the size of the bullae identified by HRCT, we have a protocol to determine an indication for the conventional or a new VATS procedure.
If the size of the bullae was greater than 2 cm, we selected the conventional VATS procedure using thoracoscopy and an endo-stapler with a 10-mm diameter by both stapler bullectomy and laser ablation to the bullous lesions surrounding the bulla.
If the size of the bullae was ≤2 cm, we performed a new VATS procedure using needle shaped thoracoscopy and endoscopic equipment of 2-mm diameter (needle VATS). We performed laser ablation of bullae alone in those patients because we had experienced that laser treatment for small bullae were useful.Informed consent was obtained in all patients according to the IRB guideline of our hospital.
2.2 The technique of the conventional VATS for PSP with bullae greater than 2 cm in size
After induction of general anesthesia, all procedures were performed under single lung ventilation with double-lumen endotracheal intubation to allow the collapse of the lung on the operated side. The patients were placed in a full lateral position and prepared as for thoracotomy. The trocar for a 0° rigid thoracoscope of 10 mm in diameter (Karl Storz Inc., Germany) was usually positioned at the sixth intercostal space on the midaxillary line. After exploration of the pleural cavity, the other two trocars, 5 and 10 mm in diameter, were inserted under endoscopic control. A 5-mm working port was placed at the fifth intercostal space on the anterior axillary line for the endoscopic grasper, and the 10-mm port at the seventh intercostal space on the posterior axillary line for the endoscopic stapling device (Fig. 1 A). A chest tube previously inserted on admission was removed. Many cases generally required two working ports in addition to the camera port. If the adhesions of the lung to parietal pleura were found, they were dissected with fingers. We used re-usable instruments for endoscopic surgery. All lobes were thoroughly examined with particular attention paid to the apex and the superior segment of the lower lobe. All identifiable bullae were usually treated using an endoscopic stapling device with a 10-mm diameter (Ethicon, Cincinnati, OH) through the surgical ports. After bullectomy, the non-contact neodymium: yttrium–aluminum-garnet (Nd: YAG) laser ablation was applied to bullous lesions surrounding the bullae. Relapse of pneumothorax was often caused by new formed bulla surrounding stapler line, so we used a laser not on the stapling line but around it.
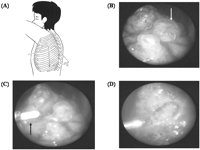
Patient in the lateral position, with placement of the three 2-mm ports. (B) Endoscopic view showing bullae (arrow) less than 2 cm in size on the apex. (C) Endoscopic ablation using Nd: YAG laser fiber (arrow) for bullae. (D) The disappearance of bullae. Schema of the port placement.
The non-contact Nd: YAG laser beam was generated by a special system (Laser Scope Corp., San Jose, CA). The power level of the laser equipment was set at a low level (8–10 W). The tissue reaction was monitored through the thoracoscope with gradually increased power levels. After bullectomy and laser ablation, we tested for air leakage with a sealing test using airway pressure.
A chest tube was inserted for drainage instead of the 5-mm port. The lung was expanded under positive pressure and the other two ports were removed. Then the skin incisions were closed.
2.3 The technique of needle VATS for PSP with bullae less than 2 cm in size
The equipment consisted of a thoracoscope and a trocar (scope sheath) with a 2-mm diameter (2MM MiniSite, United States Surgical Corp., Norwalk, CN), a digital monitor system, and standard endoscopic instruments.
Under single lung ventilation with double-lumen endotracheal intubation, the 2-mm port for needle thoracoscopy was inserted at the same position after removing the inserted chest tube. All patients generally required two working ports in addition to the camera port. The other two ports, also 2 mm in diameter, for the endoscopic grasper and the laser fiber was inserted at the third and fifth intercostal space on the anterior and posterior axillary line under endoscopic control, respectively (Fig. 1A). Identification of the bulla was confirmed through endoscopic observation. All identifiable bullae underwent the ablation with the non-contact Nd: YAG laser beam through the 2-mm surgical ports. The suitable trocar was selected for ablating pleura most effectively. The power of the laser equipment was set at a low level (8–10 W). The power level was then gradually increased. Endoscopic laser ablation of bullae was continued until they were shrunk thoroughly. Moreover, laser ablation was added to bullous lesions surrounding the bullae to prevent the formation of new bullae (Fig. 1B–D). At the disappearance of the bullae and the contraction of bullous tissue, the laser treatment was considered complete.
The tip of the 2-mm chest tube was positioned at the apex of the pleural cavity through the initial port. The absence of bleeding from the ablation area was confirmed and the anesthesiologist inflated the lung. As the lung was re-expanded, the ablated surface was less flexible than the surrounding lung parenchyma. Air in the pleural space was expelled through the lumen of the 2-mm chest tube, and the thoracoscope itself was then removed from the scope port. The wound was closed with sterile surgical tape.
3 Results
We treated 114 consecutive patients with PSP by endoscopic surgery in our institution. All patients had plain chest radiography and HRCT of the lung. A total of 101 patients (89%) had visible bullae on the apex of the lung or the superior segment of the lower lobe on the radiological examination. A bulla or bullae were found in 109 (95%) of 114 patients during thoracoscopic surgery. All of five patients without bulla or bullae belonged to needle VATS group and had moderate emphysematous lesion at the apex. They underwent laser ablation of the pleura around the emphysematous lesion.
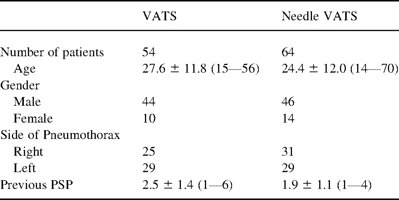
The conventional VATS was performed in 54 patients (47%) and needle VATS in 60 patients (53%). Table 1 shows patient’ profiles in both groups. We used an 8-mm chest tube in the patients of the VATS group and a 2-mm one in the Needle VATS group.
The profiles of patients with PSP treated by the endoscopic surgery
We diagnosed the eldest patient of 70 years old as spontaneous pneumothorax because he had no bulla and emphysematous lesion as far as we could determine through chest roentgenogram and computed tomography. Seventy-seven patients suffered from previous pneumothorax treated in our hospital or other institutes.
The mean operation time was 56±22 min in the VATS group and 39±17 min in the needle VATS group (Fig. 2 A). In the needle VATS group, the operation time was significantly shorter than that of the VATS group (P<0.001). The number of staples used was 3.2±1.6 (range 1–5) in the VATS group. The postoperative duration of chest drainage after surgery was on average 2.6±1.2 days (range 1–5 days) in the VATS group and 1.9±0.8 days (range 1–4 days) in the needle VATS group (Fig. 2B). In the VATS group, the mean hospital stay after the operation was 4.1±1.7 days. In the needle VATS group, the hospital stay was 3.6±1.7 days (Fig. 2C).
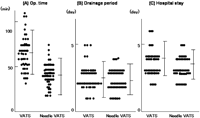
The post-operative course after both VATS and needle VATS procedures. (A) Operation time. (B) Drainage. (C) Hospital stays.
In the VATS group, usage of non-steroidal anti-inflammatory drugs, or diclofenac sodium suppository for postoperative wound pain was in 30 patients (56%). In the needle VATS group, usage of this drug was only in two patients (3%) (Table 2) .
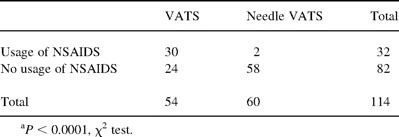
Usage of non-steroidal anti-inflammatory drugs after VATS and needle VATSa
Fig. 3 shows the changes in the CPK levels in serum with time for the pre- and post-operation days. We used a non-intra muscular injection but oral medicine or suppository for post operative pain control not to confuse the analysis of CPK. The CPK level in serum before the operation was 203±52 IU/ml. In the VATS group, the CPK level was 621±94 IU/ml 1 day after the operation, 412±98 IU/ml at 2 days, and 314±108 IU/ml at 3 days, respectively. However, in the needle VATS group, the CPK level was 316±109 IU/ml at 1 day, 274±83 IU/ml at 2 days, and 221±57 IU/ml at 3 days, respectively.
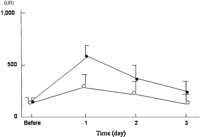
The changes in the creatine phosphokinase (CPK) levels in serum with time of the pre- and post-operation days in patients in both the VATS group (closed circle) and the needle VATS group (open circle).
There was no operative or hospital death and no patient had any major complications in the present study. No patient in either group was converted to an open thoracotomy. There were four minor complications (3.5%) including prolonged air leakage of more than 3 days in three patients and refractory intercostal pain in one patient in the VATS group, but no minor complications were noted in the needle VATS group.
Patients were followed-up for 4–45 months. Patients were examined with chest roentgenogram 1 month after discharge from our hospital. In addition, an interview by telephone was performed before writing this article.
Post-operative ipsilateral recurrence of pneumothorax was observed in four patients. Fig. 4 shows no difference between the VATS and the Needle VATS about recurrent rate by Kaplan–Meier method. The period from operation to recurrence ranged from 6 to 10 months in the VATS group and 8–14 months in the needle VATS group. All patients with recurrent pneumothorax were re-operated. The causes of recurrence were newly formed bullae on the surface of the upper lobe and the lower lobe (S6) in one patient, respectively, in the VATS group, and the upper lobe in two patients in the needle VATS group.
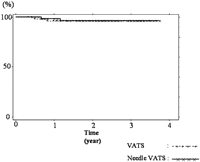
No significant difference between the VATS and the Needle VATS about recurrent rate was identified by Kaplan–Meier method.
4 Comment
The most optimal therapy for first pneumothorax was conservative therapy including observation and/or chest drainage. There were 37 patients with first pneumothorax underwent VATS in our series, which may be considered strange. But most of the patients with ‘first’ spontaneous pneumothorax had experienced weak chest pain or dyspnea for a short period in their lives and they were merely diagnosed for the first time as this disease by physical examinations and chest roentgenograms. Hence, we did not consider the number of episodes very important.
In addition, the accuracy of radiological examinations has been improved recently, but we often find small bullas and/or moderate bullous lesion of lung during VATS procedure in patients with normal CT findings. Indeed the chest drainage therapy was suitable for the patients with the ‘first’ spontaneous pneumothorax without consistent air leakage and obvious bulla on radiological examinations, but only chest drainage therapy with or without chemical pleurodesis had a relatively high recurrent rate reported as 34–65% in 1.5 years [9,10]. This is the reason why we are not particular about the results of CT in deciding the indication for surgical therapy.
The conventional VATS has usually been used as a less invasive technique than conventional thoracotomy [1,3–6]. However, in our present study, usage of non-steroidal anti-inflammatory drugs, or diclofenac sodium suppository, for postoperative wound pain was in 30 patients (56%) received the conventional VATS. Because the conventional VATS procedure was usually been performed through ports for the camera, endostapler, and grasper of 5—10 mm in diameter, which caused surgical trauma [1,3,6,8]. Of course less invasive procedures do not always lead to less surgical trauma, but we consider the reason of wound pain after VATS was caused by excessive stress during operative procedure to costal nerve via large sized trocar. Hence, we applied needle scope for VATS to reduce post operative pain.
The 2-mm port in which the length of the skin incisions is smaller than conventional VATS, is popular as the smallest one available. A smaller length of skin incision reduces the surgical trauma. The final development of a less invasive technique is the so-called needlescopic surgery, as reported in the present study.
Needlescopic surgery has the merits of not only post-operative wound pain but also cosmetic problems. The length of the wound is also a matter of great importance for beautification. The technique of needle VATS allowed wound healing without a scar and improved the patient's appearance.
We used a chest drainage tube 2 mm in diameter for the patients in this group. This small device was very effective for spontaneous pneumothorax without consistent air leakage. Chest roentgenogram taken about 1 h after chest drainage revealed a remarkable improvement of lung expansion in most patients.
The needle VATS is easily performed under a clear and magnified visual field within a short operation time. Although the laser ablation process produced a little bit of white smoke, a clear magnified view on the monitor through the needle thoracoscope can be maintained thoroughly. There is a potential risk in performing this procedure that the laser ablation of the bullae and the surrounding tissue of the bullae may increase the possibility of bleeding or alveolar air leakage.Indeed, YAG laser had haemostatic effects by moderate abrasion, but over abrasion induced pulmonary parenchyma injury, which caused severe bleeding and prolonged air leakage. However, the present results showed that such a risk was low.
The recurrence rate of pneumothorax with bullae less than 2 cm in size after performing needle VATS was lower than those reported in the previous conventional endoscopic techniques with standard equipment. The reported incidences of recurrence after a conventional VATS had been 2.7–11.5% [1,3–6,8]. Low post-operative recurrence rates have been widely recognized below 1% after conventional thoracotomy with resection of bullae and/or pleurodesis for PSP [11–14]. It was difficult to identify the reason why the recurrence rate after conventional VATS was high. It has been suggested to be due to overlooking bullae or to the formation of new bullae on the bullous lesions surrounding the staple row. The detection of bullae responsible for this episode of pneumothorax may be more difficult than conventional thoracotomy because the lung is deflated during VATS.
It has been reported that pleurodesis or pleurectomy plays an important role in preventing the recurrence of pneumothorax after VATS [4,11,12,15]. Naunheim recommended additional parietal pleurectomy for the patient with PSP [4]. Our experience of PSP suggests that it may be as useful for the prevention of recurrence to treat the bullous parenchyma lesion surrounding the bullae with laser ablation as chemical or mechanical pleurodesis or pleurectomy.
In the needle VATS group, the CPK level in serum, the indicator of muscle damage, was lower. Therefore, usage of non-steroidal anti-inflammatory drugs for post-operative wound pain could be reduced. We moreover primarily evaluated the incidence of post-operative complications and secondarily the duration of the hospital stay in either group. In the present series, no patient was converted to open thoracotomy due to intraoperative complications. However, three patients (5%) in the conventional VATS group had air leakage continued for 3 days or longer post-operatively, probably because they had more extensive disease of bullae and emphysematous lesions. Reoperation due to post-operative complications was 0% in either group. This shows that the procedure of needle VATS is as satisfactorily safe as that of conventional VATS. We have applied needle VATS for spontaneous pneumothorax encouraged by these data in these years and want to continue analyzing recurrent rate and prognosis of this novel technique.
5 Conclusion
Needle VATS is useful, safe, and less invasive for the treatment of PSP. Moreover, in the future, we hope the application of this new technology will spread for patients with bullae greater than 2 cm in size.




