-
PDF
- Split View
-
Views
-
Cite
Cite
William E. Cohn, Marc Ruel, Jian Ping Zhang, Frank W. Sellke, Robert G. Johnson, Internal thoracic artery flow competition: studies in a canine H-graft model, European Journal of Cardio-Thoracic Surgery, Volume 23, Issue 1, January 2003, Pages 56–59, https://doi.org/10.1016/S1010-7940(02)00656-5
Close - Share Icon Share
Abstract
Objective: Internal thoracic artery (ITA) flow competition is a diversion of graft flow through intact ITA branches with a net decrease in perfusion to the grafted coronary. Although a widely acknowledged phenomenon, the conditions under which flow competition occurs have not been established. This is examined in a canine H-graft model. Methods: Eight dogs had a right ITA segment interposed (H-graft) between their in situ left ITA (LITA) and the snared left anterior descending (LAD) coronary artery. Proximal LITA and H-graft flows were measured at baseline and during pacing-induced tachycardia, phenylephrine-induced hypertension, and nitroprusside-induced hypotension. Flows were measured with the distal LITA open and occluded. Two additional animals were subjected to eight separate 2-min periods of LAD ischemia, after which post-ischemic H-graft flow measurements were obtained with and without distal LIMA occlusion. Results: During baseline conditions, proximal LITA flow was greater when the distal LITA was open rather than occluded (46±15 versus 35±12 ml/min, respectively; P=0.002), but H-graft flow did not change significantly (32±18 versus 35±18 ml/min, respectively; P=0.21). Similarly, occlusion of the distal LITA had no impact on H-graft flow during tachycardia, hypertension, or hypotension. Only in animals subjected to transient LAD ischemia did H-graft flow increase with distal LITA occlusion, albeit marginally (65±7–70±9 ml/min, occluded versus open, respectively; P=0.04). Conclusions: Mild diversion of flow from the LAD was demonstrated during immediate post-ischemic coronary reperfusion only, and could not be elicited under any other physiologic condition. These data suggest that flow competition is unlikely to constitute a clinically significant limitation to the use of H-grafts or other modalities that leave ITA branches patent.
1 Introduction
Internal thoracic artery (ITA) flow competition is a diversion of graft flow through intact ITA branches with a net decrease in flow to the grafted coronary. It has been suggested that patent medium-sized branches from the ITA may inherently result in flow diversion [1–4]; however, this remains controversial and the conditions under which this phenomenon occurs have not been established. The issue becomes increasingly relevant as some new minimally invasive cardiac revascularization strategies involve leaving intercostal and distal ITA branches intact [5–8].
We have previously described the clinical use of an alternative minimally invasive direct coronary artery bypass grafting technique termed the H-graft [9]. The H-graft is a short segment of conduit, either artery or vein, interposed between the side of the in situ left ITA (LITA) and the side of the left anterior descending (LAD) coronary artery (Fig. 1) . All LITA side branches are left intact. One may also choose to ligate the LITA distal to the origin of the H-graft, or leave it patent in order to preserve future surgical options; however, the possibility of flow diversion away from the coronary artery exists if the LITA distal to the origin of the H-graft is left open.
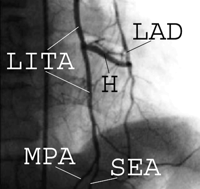
Selective left internal thoracic angiogram showing an H-graft at one week postoperatively. In this patient, the intercostal branches and distal LITA were kept patent. H=H-graft (using a right inferior epigastric artery segment); LAD=left anterior descending artery; LITA=left internal thoracic artery; MPA=musculophrenic artery; and SEA=superior epigastric artery.
We designed a canine model of H-grafting in order to examine the ITA flow competition hypothesis, which dictates that an increase in H-graft flow down the coronary artery would occur with distal LITA occlusion. The model involved various controlled physiologic conditions and post-ischemic reperfusion, in which the perfusion pressure as well as the resistance of the coronary and skeletal muscle beds were altered.
2 Methods
2.1 Animal preparation
Eight mongrel dogs (13–22 kg) had anesthesia induced by an intravenous barbiturate injection, followed by intravenous 80 mg/kg alpha-chloralose and 500 mg/kg urethane. The animals were intubated endotracheally, mechanically ventilated, and their arterial blood gases recorded every 30 min. Femoral arterial pressure, heart rate, and electrocardiogram were continuously monitored. Sternotomy was performed and 200 U/kg of heparin was administered. Pacing wires were attached to the right atrium and connected to an external pulse generator.
The right ITA (RITA) was dissected from the chest wall and prepared as a free graft. The RITA was then interposed as an H-graft between the side of the in situ LITA and the LAD, with the coronary anastomosis performed on the beating heart using a stabilizer (Genzyme, Fall River, MA). Doppler flow probes (Flowsonics, Redwood, CA) were placed around the LITA just proximal to the origin of the H-graft and on the H-graft itself (Fig. 2) . The probes were calibrated and their readings continuously recorded.
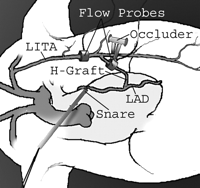
Schematics of the set-up used for the experiments. LAD=left anterior descending artery; and LITA=left internal thoracic artery.
All animals received humane care in compliance with the Beth Israel Deaconess Medical Center Animal Care and Use Committee and the National Research Council's Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animals and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985).
2.2 Experimental protocol
2.2.1 Physiologic conditions
A 4-0 polypropylene suture was snared around the proximal LAD and the animal was allowed to stabilize for 15 min. LITA and H-graft flows were measured in this baseline state, and subsequently during stress conditions of tachycardia, hypertension, and hypotension. Tachycardia was achieved by right atrial pacing at a rate 30% above the baseline rate. Hypertension was accomplished by titrating an intravenous infusion of phenylephrine to increase the mean arterial pressure (MAP) to 30% above baseline. Hypotension was induced with sodium nitroprusside to decrease the MAP by 30% compared to baseline values. Each hemodynamic intervention took no more than 4 min to achieve the targeted parameter, and stability was confirmed for 5 min before measuring flows. Two sets of flow measurements were made for each physiologic state; one with the distal LITA open, and one with the distal LITA temporarily occluded with an atraumatic spring-loaded vascular clip (Fig. 2). An increase in H-graft flow when the distal LITA was temporarily occluded was considered potential evidence that flow diversion was taking place, and that the graft flow supply may not have been meeting the coronary arterial demand under this particular physiologic condition. The LAD snare was released after each physiologic stress state, and myocardial perfusion was allowed to return to baseline before proceeding with the next state.
2.2.2 Post-ischemic reperfusion
Two additional animals were subjected to eight separate 2 min periods of severe anterior myocardial ischemia followed by flow measurements after each episode, with and without distal LITA occlusion. Anterior myocardial ischemia was created by simultaneously snaring the proximal LAD and occluding the H-graft, in order to achieve a maximal, albeit transient increase in myocardial demand and coronary vasodilatation associated with post-ischemic reperfusion. Euthanasia was carried out at the end of the protocol with 10 ml/kg of a saturated KCl solution administered intravenously.
2.3 Data analysis
Flows for each intervention are reported as mean±standard deviation. Within each experimental condition, a paired Student's t-test was used to compare the values obtained at each flow probe site with the distal LITA open versus occluded. Bonferroni corrections were performed when appropriate. All tests were 2-tailed, and P values of less than 0.05 were considered statistically significant.
3 Results
3.1 Physiologic conditions
Flow measurements at baseline and under three controlled physiologic conditions are shown in Table 1 . Although the mean proximal LITA flow was greater when the distal LITA was open compared to when it was closed, in none of these conditions did H-graft flow increase significantly with distal LITA occlusion. Under the various conditions tested, the flow through the distal LITA, when open, represented 18–30% of the proximal LITA flow. Nevertheless, since H-graft flow did not increase with occlusion of the distal LITA under any of the three physiologic conditions, the inflow reserve capacity of the proximal LITA appeared sufficient to meet the perfusion demands of the anterior myocardial territory even with the distal LITA open.

Flow measurements at baseline and under three physiologic conditions
3.2 Post-ischemic reperfusion
In contrast to animals subjected to controlled physiologic conditions, dogs subjected to 2 min of complete LAD ischemia did demonstrate an increase in H-graft flow as a result of distal LITA occlusion. With the distal LITA open the flow was 65.1±7.1 ml/min, and increased by 7% to 69.7±8.8 ml/min during distal LITA occlusion (P=0.04). Regardless of whether the LITA was open or occluded, total H-graft flow was also significantly increased after ischemia compared to other physiologic states (65.1±7.1 and 69.7±8.8 ml/min versus Table 1 flows, open and occluded distal LITA, respectively; P<0.05 versus baseline, tachycardic, hypertensive, and hypotensive conditions).
4 Discussion
Under the controlled physiologic conditions tested in this study, H-graft flow was independent of distal LITA patency and flow. Occlusion of the distal LITA only resulted in decreased proximal LITA flow, while the demand through the H-graft and open distal LITA was adequately met by the flow reserve of the proximal LITA, possibly enabled by the predominant diastolic and systolic flow patterns of the coronary and systolic circulations, respectively. Since the distal LITA is, in comparison to anterior intercostal branches, the largest ITA side branch with a potential to divert flow away from the coronary circulation, one may therefore infer that diversion of H-graft flow from ITA branches is unlikely to represent a clinically significant phenomenon, at least under acute physiologic conditions.
A mild degree of diversion was however observed after post-ischemic coronary reperfusion. This temporary condition of maximal coronary vasodilatation was associated with the proximal LITA delivering more flow to the coronary bed via the H-graft when the distal LITA was occluded than when it was open. We believe that this finding may reflect an acute physiologic limitation in proximal LITA flow capacity to provide adequate perfusion when the demand is greatest in the coronary bed and a competitive outflow is also present (i.e. open distal LITA).
It is likely that our results showing absence of flow diversion despite retained LITA branches and an open distal LITA are predicated on the maintenance of adequate proximal LITA flow. In the presence of a flow-limiting subclavian artery or proximal LITA stenosis, it is probable that flow diversion would occur under the conditions created in these experiments. This is suggested by clinical reports of one type of LITA flow competition in which proximal left subclavian stenosis in patients with LITA grafting to the LAD has resulted in a steal syndrome [10,11]. In this particular scenario, the diverting bed is the left upper extremity, with proximal graft flow so limited by an upstream stenosis and a diverting bed so large that LITA graft flow is retrograde (i.e. from the heart to the upper extremity), and results in severe myocardial ischemia.
While our experiments demonstrated that flow diversion from the LAD to the LITA does not take place under controlled physiologic conditions involving a normal coronary outflow bed, there are limitations to the extrapolation of these data. Our experiments were not designed to examine the time-course and duration of flow changes, and a chronic ischemic model was not studied. Furthermore, factors related to the chronic dilatation and maturation of ITA grafts or to the presence of diffuse distal coronary disease were not modeled. Nevertheless, this study indicates that acute ITA steal due to retained LITA branches constitutes an unlikely phenomenon in the presence of normal proximal ITA inflow and a normal distal coronary circulation, and therefore may not represent a clinically significant limitation to the use of H-grafts or other modalities that leave LITA branches patent.
Drs Cohn and Ruel were supported in part by a grant from the Harvard Center for Minimally Invasive Surgery for this study.




