-
PDF
- Split View
-
Views
-
Cite
Cite
Younes Boudjemline, Damien Bonnet, Gabriella Agnoletti, Pascal Vouhé, Aneurysm of the right ventricular outflow following bovine valved venous conduit insertion, European Journal of Cardio-Thoracic Surgery, Volume 23, Issue 1, January 2003, Pages 122–124, https://doi.org/10.1016/S1010-7940(02)00660-7
Close - Share Icon Share
Abstract
A case of aneurysm of the right ventricular outflow tract is described after repair of tetralogy of Fallot using a Contegra supported conduit. Angiograms revealed that the aneurysm was located between the ventricular anastomosis and the proximal ring of the conduit confirming echocardiographic data. Because the conduit between the rings was not dilated, the valve was perfectly functioning. Pulmonary anastomosis was severely stenosed explaining the dilatation seen below. Conduit replacement with resection of the aneurysmal part of the failing conduit was performed. Supported conduits do not eliminate the risk of secondary dilatation below the artificial ring but preserve valvular function.
1 Introduction
A variety of congenital heart defects involving the right ventricular outflow tract (RVOT), namely tetralogy of Fallot with or without pulmonary atresia, truncus arteriosus, or complex transposition of the great arteries require surgical repair during infancy. Current techniques consist of the repair of intracardiac anomalies associated with the interposition of a conduit between the right ventricle (RV) and the pulmonary arteries (PA). Various conduits housing or not housing a biological valve have been used to reconstruct the RVOT [1–4]. Recently, a totally integrated valved conduit has been introduced in clinical practice. It consists of a bovine jugular vein naturally having a valve with sinuses (Contegra™, Medtronic). Conduits are available supported or unsupported, in various sizes from 12 to 22 mm. In the supported model, two external cloth-covered propylene rings are added to provide additional support to the valve on either of its sides. Data concerning the life span of these conduits are limited [5–8]. However, since its first introduction, no conduit failure has been reported. Here, we describe a severe dilatation of a Contegra conduit in a 5-year-old boy leading to its premature replacement.
2 Case report
A 5-year-old boy had a history of tetralogy of Fallot with pulmonary atresia, multiple major aorto to pulmonary collateral arteries (MAPCAs) and confluent PA. At the age of 4 months, he underwent unifocalization of the right MAPCAs on a 4 mm Blalock Taussig shunt. Two months later, a left MAPCA that compressed the bronchial tree was ligated via a left thoracotomy. In March 1999, because of persistent hypoplastic pulmonary vessels, he had as a third palliation an RV to PA opening with the interposition of a 6 mm Gore-Tex conduit. At that time, the ventricular septal defect (VSD) was left opened. Two years later, he underwent the complete repair of his cardiac malformation. A 14 mm supported Contegra conduit (Medtronic) was placed from the RV to the PA bifurcation using running sutures of absorbable thread, without any addition of foreign material nor trimming of extremities of the conduit. The VSD was closed using a patch of heterologous pericardium. During operation, right and left PA were respectively measured at 8 and 6 mm. Sequential echocardiographs showed a progressive increase of RV pressure with a gradient over the conduit. No valvular insufficiency was seen on Doppler study. Because of poor echogenicity, RVOT anatomy and valve motion could not be precisely assessed. Cardiac catheterization confirmed iso-systemic RV hypertension with normal PA pressure after pulmonary bifurcation. Selective angiograms of the conduit above the valve showed a long stenosis of the conduit that extended from the pulmonary anastomosis to the pulmonary bifurcation. No opacification of the RV was observed confirming the perfect function of the pulmonary valve. A more proximal contrast injection below the valve showed aneurysmal dilatation extending from the ventricular anastomosis to the first ring of the supported conduit (Fig. 1) . The conduit size between the two rings was normal. Fourteen months after its insertion, the Contegra conduit was completely retrieved (Fig. 2) . Histological examination revealed an important fibrointimal proliferation located in the area of pulmonary anastomosis. Valvular leaflets were partially colonized by host cells. A sub-valvular calcification was seen in the conjunctive tissue. A mild foreign body reaction was also present at the outside layer of the conduit.
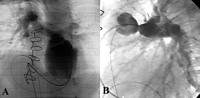
Angiograms showing (A) an aneurysmal dilatation extending from ventricular anastomosis to the proximal ring of the conduit (four chamber view), and (B) a stenosis of the pulmonary anastomosis, a non-dilated conduit and the perfect function of the valve (lateral view).
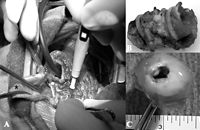
Macroscopic views during surgical retrieval of the Contegra conduit. Note the dilatation of the proximal part of the conduit (*) and the normal size between the two rings.
3 Discussion
Aneurysmal dilatation of non-supported Contegra conduits has been only rarely reported [7,8]. As shown in the present report, rings located at each end of the valve were efficient to prevent dilatation of the valve, thus preserving its function. However, the bovine vein below the inferior ring was unprotected and a dilatation of that part was observed. It is likely that stenosis of the pulmonary anastomosis was responsible for increased right ventricular pressure. Subsequently, during each systole, the RV ejected blood at high pressure increasing stress on the wall of the conduit, mostly on its non-protected part, leading to its progressive distension. Histologically, a pathological intimal proliferation was present in the area of the pulmonary anastomosis, fully explaining pulmonary obstruction. The mechanisms involved are unknown. An abrupt reduction of vascular size, at the end of the conduit and in the beginning of PA branches, creates a pressure gradient and a non-laminar accelerated blood flow that may increase shear stress and stimulate locally an exaggerated intimal proliferation. Since stenosis of the PA bifurcation could result from a sub-optimal reconstruction, particular care should be taken to avoid further stenosis when anastomosing PA to the conduit, and so in particular in patients with hypoplastic PA. The presence of inflammatory tissue underlines the possibility of an immunologic rejection. However, this tissue was uniformly present along the xenograft on the outside layer and did not explain the obstruction of the conduit. Chemical interactions between suture material and glutaraldehyde-fixed conduit, cellular damage during suturing, and direct cytotoxicity of cross-linking agents might enhance intimal hyperplasia initiated by haemodynamic turbulence. If correct, this hypothesis must lead to a modification of our practice. Firstly, the insertion of a Contegra conduit in patients with high RV pressures resulting from pulmonary hypertension or pulmonary stenosis should be done with caution if high RV pressures are expected to fall or contraindicated otherwise because of the risk of abnormal dilatation of the venous wall. Secondly, conduit obstruction is usually neglected as long as RV pressure is inferior to 75% of systemic pressure and/or RV function is not altered. Patients with stenotic conduits are thus followed for years before an indication to conduit replacement is made. With regard to possible inappropriate dilatation of Contegra conduits, closer follow-up is mandatory and PA stenosis should probably be treated more energetically to avoid such a complication. Moreover, the occurrence of a pulmonary leak in the presence of PA stenosis enhances the volume of blood that is regurgitated in the RV leading to a more rapid heart failure. Synthetic rings in supported conduits avoid dilatation of the valve and thus preserve its function. This would make the use of supported conduit preferable.




