-
PDF
- Split View
-
Views
-
Cite
Cite
Duilio Divisi, Carmelo Battaglia, William Di Francescantonio, Guido Torresini, Roberto Crisci, Giant bullous emphysema resection by VATS. Analysis of laser and stapler techniques, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 6, December 2002, Pages 990–994, https://doi.org/10.1016/S1010-7940(02)00567-5
Close - Share Icon Share
Abstract
Objective: Advances in video-assisted thoracic surgical (VATS) technique led the authors to reconsider the treatment and thoracoscopic management of patients with giant bullous emphysema (GBE). Methods: From January 1993 to December 2001 we treated 40 patients with unilateral GBE: 24 males and 16 females, mean age 51±1 years. Thirty patients presented respiratory insufficiency, seven patients a spontaneous pneumothorax and three patients a bullae infection. Excision was performed by using Nd:YAG laser in five patients (12.5%) and stapling device in 35 patients (87.5%). Among the last 35, in 20 patients a partial pleurectomy stripping up to the 5th intercostal space was associated. In 15 patients this technique was modified through the systematic application of polytetrafluoroethylene (PTFE) to reinforce stitches. Results: We experienced one conversion to open thoracotomy owing to haemorrhaging, in one patient who underwent a partial pleurectomy stripping. In the stapler resection patients, with PTFE application, the mean duration of air leaks, for type 1 bullae of Wakabayashi was 2.2±1.8 days and, for type 4, 5.9±1.4 days; the mean length of hospital stay was 6.1±0.5 days. Conclusions: The resection in VATS of giant bullous emphysema by stapling device associated to reinforcement in PTFE reduces duration of air leaks and hospitalisation and improves pulmonary function.
1 Introduction
Bullous emphysema is an expansion of the alveolar spaces with a diameter over 1 cm and a wall thickness inferior to 1 mm [1]. Giant bullous emphysema (GBE) is a rare pathology. Surgical intervention is indicated in the event of progressive respiratory insufficiency and/or recurring or persistent pneumothorax, of hemoptysis, and a state of infection of the bulla [2]. The aims of this treatment are: (a) a reduction in compression exercised by the bullae on the adjacent pulmonary parenchyma, on the diaphragm and the mediastinum; (b) optimisation of the ventilation-perfusion relationship; and (c) a reduction of dead spaces in case of extended communication between the bronchial terminals. Best functional results are obtained in bullae with a volume superior to 1/3 of the hemothorax [3]. Advances in video-assisted thoracic techniques (VATS) which are less invasive compared to thoracotomy [4] and sternotomy [5], have led to the development of various endoscopic methodologies aimed at improving the clinical-functional situation of the patient and reducing the length of hospital stay. The aim of this study was to assess the quality of laser and stapler resections with or without polytetrafluoroethylene (PTFE) to reinforce lung staple lines in giant bullous emphysema.
2 Patients and methods
From January 1993 to December 2001, we observed 40 patients affected by monolateral GBE (Fig. 1) : 24 males (60%) and 16 females (40%) with an average age of 51±1 years (extremes: 25–63 years). All patients underwent pre-operative testing consisting in: (a) general evaluation (hemochrome with leukocyte formulas and electrolytes, liver function); (b) respiratory (spirometry, blood gas analysis, perfusion and ventilation scintigraphy); (c) cardiac (electrocardiogram, echocardiography with measurement of pulmonary pressure); and (d) radiological (lung radiography, high-density computerized tomography, CT, of the thorax). Thirty patients (75%) revealed signs of respiratory insufficiency (15 with stage III dyspnea, ten with stage IV dyspnea and five with stage V dyspnea) according to Sadoul [6] (Table 1) , seven patients (17.5%) with spontaneous pneumothorax and three patients (7.5%) with an infection of the bulla. The classification of emphysema bullae employed was that of Wakabayashi [7] (Table 2) .
Giant bulla in left base with bullous dystrophy in the right base of pulmonary parenchyma.


2.1 Surgical technique
A selective intubation was performed under general anaesthesia. Cardio-respiratory parameters were carefully monitored. The patients were placed in the standard thoracoscopic position. Video-thoracoscopic equipment used is described in Table 3 . Three trocars were positioned in a triangle at the base of the bulla to facilitate dissection and resection. The topographical aspects may initially require a small incision in the bulla wall (YAG scalpel or coagulating scissors according to the technique used) with resulting collapse of the parenchyma. The procedure was carried out on five patients (12.5%) with an ND:YAG laser (SMART 1064)1, 40–100 W max with pulses of 0.1–99.9 s. This technique has been described by Sharpe et al. [8]. In 35 patients (87.5%) resection of the bulla was performed with a linear stapler device: Endo GIA 30 (Autosuture) and/or Endo-path 45 (Ethicon). Among the group of 35, a partial pleurectomy stripping up to the 5th intercostal space was associated in 20 cases (57.2%). In 15 patients (42.8%) this technique was modified by the application of polytetrafluoroethylene strips to reinforce the stitches, without pleurectomy (Fig. 2) . Data relative to length of anaesthesia and surgical time are summarised in Table 4 .


All patients were extubated in immediate post-operative time.
2.2 Statistical analysis
In ten progressive respiratory insufficiency patients treated with a stapling device associated to PTFE, the statistical analysis was performed with SPSS (Windows release 6.1). Data were expressed as mean±standard deviation and 95% confidence interval and the statistical significance was estimated by Student's t-test for unpaired data. P values <0.05 were considered significant. Group multiple comparisons of parametric data were performed by Kurs-K method.
3 Results
No operative or post-operative death was documented nor was post-operative ventilator support required. We experienced one conversion to open thoracotomy (2.5%) owing to peri-operative haemorrhaging of intercostal arteries and vena cava, in one patient who underwent a partial pleurectomy stripping; the hemostasis carried out with clips and continuous suture. In the laser patients, the mean duration of air leaks, for type 1 bullae of Wakabayashi, was 4.6±2.3 days and, for type 4, 15.2±10.1 days; the mean length of hospital stay was 16.3±3.5 days. In the stapler resection patients, the mean duration of air leaks without PTFE application, for type 1 bullae of Wakabayashi, was 3.8±0.5 days and, for type 4, 10.5±4.6 days; the mean length of hospital stay was 12.7±5.8 days. The mean duration of air leaks with PTFE application, for type 1 bullae of Wakabayashi, was 2.2±1.8 days and, for type 4, 5.9±1.4 days; the mean length of hospital stay was 6.1±0.5 days. Twenty-six (86.6%) progressive respiratory insufficiency (PRI) patients treated with linear stapler device (another four PRI patients were treated with laser), showed an improvement in dyspnea and respiratory function. In ten patients who underwent a stapler resection associated to a PTFE, improvement in respiratory parameters remained constant over a 12 month period following intervention (Table 5) . Stapler resection was successful in 100% of pneumothorax (Pnx) patients. In the 36 months following the operation, no occurrence of Pnx was observed. Histological examination was performed in all cases, showing an expansion of air spaces associated with fibrosis and/or inflamed infiltration.
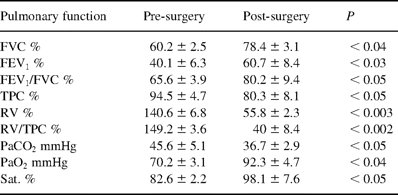
Pre-and post-surgery (12 month) functional parameters in ten progressive respiratory insufficiency patients treated by PTFE
4 Discussion
Our study showed that thoracoscopic resection of giant bullae is a safe, easy and effective alternative to the conventional open technique. This surgical technique is justified even in cases of severe respiratory insufficiency, rendering the mini-invasive treatment of intracavity drainage proposed by Macarthur and Fountain [9], although modified by Uyama et al. [10] by introduction into the lung of OK-432 and fibrin glue, a less likely alternative. Despite a satisfactory functional result with an increase of 17.6% in FVC and 32% in FEV1, the authors recorded an elevated risk in bronchopneumonia and a delayed removal of drainage (after 40 days) [10].
The results of endoscopic treatment depend on the selection of patients and the type of operating method used. Pulmonary function tests are fundamental but high-density CT permits an accurate definition of: (a) site, volume and extension of the bulla; (b) presence of associated bullae; and (c) quality of adjacent lung parenchyma [11,12]. As for the therapeutic approach, literature has shown the relative ease in execution and effectiveness of treatment by video-assisted thoracoscopy [13,14], thus making it the chosen method in the removal of giant bullae. Consequently, it must be said that the problem tends to reside in the type of endoscopic technique to be adopted. In a series of five patients, Hillerdal et al. [15] proposed the simultaneous application of both fibrin glue and thrombin in the bulla. Functional results proved satisfactory due to the 33.3% increase in VC and the 22.2% increase in FEV1 and 10.7% decrease in RV. Shinonaga et al. [16], in a patient with Marfan's Syndrome, resected a giant bulla by an incision of the wall and looped ligation of the excessive wall of the bulla with Endo-GIA at its base.
Our experience shows that the methods to be adopted are twofold: laser and linear stapler device. The laser method however, produced less comforting results as to the length of anaesthesia, post-operative air leaks and length of hospital stay in relationship to resection by stapler. These results compare favourably to those obtained by Wakabayashi [7], who in a series of 16 patients treated by Carbon dioxide or Nd:YAG laser, reported an air leak time, for type 1 bullae, of 5.7±2.5 days (versus 4.6±2.3 days) and, for type 4, 17.3±15.07 days (versus 15.2±10.1 days). The author, however, had resorted to post-operative ventilator support, for type 1 bullae, 4.5±1.6 h and, for type 4 bullae, 53.8±69.4 h: an occurrence not observed in our series. Better results were obtained by Sharpe et al. [8] in average duration of hospital stay (4.1 days) and tube thoracostomy (2.1 days), in 11 patients affected by pneumothorax and successfully treated in VATS. This can be attributed to their greater experience and the fact that four out five of our patients had severe respiratory insufficiency.
A linear endoscopic stapler device developed in 1990, met with the approval of the authors in situations of spontaneous pneumothorax bullectomy thanks to the ease and speed in surgical manipulation. In a series of 37 patients with three cases of giant bullous emphysema, Yamaguchi et al. [17] proposed associating resection by stapler to suturisation of the parenchyma and by using chemical pleurodesis routinely in case no distinct bullae were observed. The resection of emphysematous lung tissue by linear stapler is associated to prolonged air leakage. Two methods are generally used to favour lung expansion: wall pleurectomy and reinforcement of the suture line. Total wall pleurectomy was proposed by Gaensler [18] in 1956. The technique involved an elevated risk of haemorrhaging and reduced diaphragmatic motility. For this reason the original method was substituted by apical pleurectomy (V–VI intercostal space) as reference point in order to carry out a definitive pleurodesis, with a resulting rate of recurrence of pneumothorax of 0.4%. Furthermore, sub-total pleurectomy slows or halts the development of bullae reinforcing lung cortex. Different materials have been employed to reinforce the stapling device in emphysematous lungs [19,20]. Cooper [21] proposed the use of bovine pericardial strips (1 cm in width and 10 cm in length) for the reduction of lung volume in emphysema. Our findings are that bovine pericardial strips are inconvenient in regards to cost (750.00 Euros a piece) and application, compared to PTFE. In our series, the successful use of polytetrafluoroethylene strips prompted us to continue this new experience, allowing a reduction in anaesthesia time (50±10 versus 62±15 min), in surgical time (25±2 versus 35±5 min), in post-operative air leakage (2.2±1.8 versus 3.8±0.5 days for type 1; 5.9±1.4 versus 10.5±4.6 days for type 4), and in length of hospital stay (6.1±0.5 versus 12.7±5.8 days). In ten patients with progressive respiratory insufficiency, the above resulted in a statistically relevant clinical-functional improvement (P<0.05) at 1 year from surgical intervention. Moreover, the use of PTFE allows for a valid pleurodesis, avoiding the risk of haemorrhage which may follow pleurectomy by stripping (2.5% in our series). Menconi et al. [22] in 29 GBE patients treated by stapler device and by loop, do not take into consideration the necessity of reinforcement focusing rather on the good results obtained in drainage time. These same authors, however, experienced two re-operations by open thoracotomy due to progressive air leakage failure and two patients died of respiratory failure.
In conclusion, the thoracoscopic technique of excision of giant bullous emphysema by linear stapling device reinforced by PTFE allows for rapid recovery of functions in patients in relation to a reduction in length of post-operative air leaks as well as hospital stay. Pre-operative radiological-functional evaluation dictates choices which optimise the cost-benefit relationship, considering the expense of polytetrafluoroethylene (121.36 Euros for two pieces).
References
DEKA, Medical Electronics Laser Associated, Calenzano, Italy.
- hemorrhage
- lasers
- length of stay
- polytetrafluoroethylene
- respiratory insufficiency
- surgical procedures, operative
- thoracic surgery, video-assisted
- thoracoscopy
- thoracotomy
- infections
- blister
- spontaneous pneumothorax
- nd-yag lasers
- pulmonary function
- medical devices
- excision
- pleurectomy
- giant bullae
- pulmonary air leakage
- intercostal space




