-
PDF
- Split View
-
Views
-
Cite
Cite
I.M. Keeling, P. Oberwalder, M. Anelli-Monti, H. Schuchlenz, U. Demel, G.P. Tilz, P. Rehak, B. Rigler, Cardiac myxomas: 24 years of experience in 49 patients, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 6, December 2002, Pages 971–977, https://doi.org/10.1016/S1010-7940(02)00592-4
Close - Share Icon Share
Abstract
Objectives: In this single-center study we reviewed our experience with a significant number of cardiac myxoma cases occurring over the past two decades. Patients and methods: Cardiac myxomas represented 86% of all surgically treated cardiac tumors at our center. Specifically, there were 49 consecutive patients, each with at least one myxoma. A detailed clinical, immunological, and echocardiographic long-term examination of 37 patients revealed one recurrent myxoma. Results: Most myxomas originated from the left atrium (87.7%), but also much less frequently from the mitral valve (6.1%), from the right atrium (4.1%), and from the left and right atria (2.0%). The myxomas produced a prolapse into the left ventricle in 40.8% of the patients, mitral stenosis in 10.2%, and threatened left ventricular outflow tract obstruction in 2.0%. Multiple myxomas were found in 20.4% of the patients. Cardiac signs appeared in 93.9% of the patients. Preoperative embolic events had occurred in 26.5%. Immunologic alterations were present in 87.5%. For resection, a bilateral atriotomy was used. An additional aortotomy was needed to expose one mitral valve myxoma. Postoperatively, 81.1% of the patients remained without cardiac symptoms. The early mortality rate was 2.0% and the late mortality rate was 6.1%. Long-term prognosis was excellent with an actuarial survival rate of 0.74. Specific immunologic alterations were found in 71.4% of the patients. The actuarial freedom from reoperation of the myxoma was 0.96. The rate of reoperations was low with 2.0% after 24 years. Conclusions: Myxomas were usually detected and operated on in symptomatic patients. A high index of suspicion seems important for early diagnosis. Immunologic findings may play an additional role in confirming the diagnosis and the recurrence of a myxoma. Immediate surgical treatment was indicated because of the high risk of embolization or of sudden cardiac death. Also, a familial genesis must be excluded in myxoma patients.
1 Introduction
Intracardiac myxoma is the most common tumor of the heart with an estimated incidence of 0.5 per million population per year [1]. Among operations with the heart–lung machine, 0.3% are resections of a cardiac myxoma [2]. Up to 80% of myxomas are localized in the left atrium, of which 75% involve the interatrial septum; 7–20% are found in the right atrium; the rest of up to 10% each are either biatrial, in the right ventricle, or in the left ventricle [2–4]. Myxomas are found emerging from valve tissue extraordinarily rarely [3]. The symptoms have varied greatly, depending on the size and the localization of this tumor. Along with characteristic features in the transesophageal echocardiography (TEE), variations from a normal immunologic state may occur in these patients [5]. We studied the pre- and postoperative pattern of immunologic changes in 39 myxoma patients including one with recurrent multiple myxomas. After up to 24 years of follow-up, treatment options and long-term clinical outcome in 49 cardiac myxoma patients are reported in this study.
2 Patients and methods
From February 1978 to February 2002, 49 patients with a cardiac myxoma were surgically treated in our center. Specifically, 54 myxomas were operated on in these 49 patients, as two patients had two myxomas and one patient had four. In fact, 0.35% of open-heart procedures were performed for cardiac myxoma. These patients included 12 males (24.5%) and 30 females (75.5%). The mean age of the patients at the time of operation was 55 years (range 22–79 years). The most frequently observed symptoms were associated with mitral valve obstruction (Table 1) . Such an obstruction caused congestive heart failure in five patients (10.2%), and 25 (51.0%) reported dyspnea. Eight (16.3%) suffered a stroke, and seven (14.3%) had transient neurologic symptoms. Two patients (4.1%) had an acute peripheral embolism, one in the caval vein and the other in the left arm artery. In addition, constitutional symptoms and signs of a generalized disease were reported. None of the patients had a family history of myxoma.
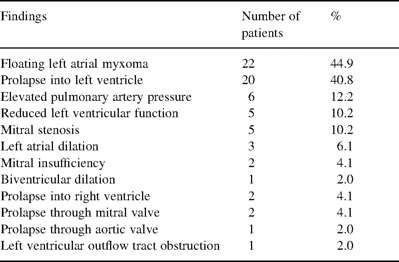
Preoperative echocardiographic findings using TTE and TEE (n=49 patients)
2.1 Diagnosis
The methods used to confirm the diagnosis of a cardiac myxoma varied during the two decades. In early years, 15 patients were diagnosed by cardiac catheterization. More recently, transthoracic echocardiography (TTE) was performed in 11 patients, TEE in 19 patients and magnetic resonance imaging (MRI) in four patients; the correct diagnosis was made in every case (Table 1). The tumors arose from the interatrial septum in 30 patients (61.2%), from other locations in the left atrium in 13 patients (26.5%), from the mitral valve in three patients (6.1%), from the right atrium in two patients (4.1%), and from both the left and right atria in one patient (2.0%). In one case, TEE revealed an intracardiac tumor at the ventricular-sided basis of the anterior mitral valve leaflet resulting in a systolic prolapse through the aortic valve and a 50% obstruction of the left ventricular outflow tract. In another rare case, the myxomas were located in both atria of the heart. MRI of the neurocranium excluded asymptomatic microembolisms of the brain.
Preoperative routine laboratory investigations in myxoma patients showed elevated levels of C-reactive protein (CRP) in 57.1% and an acceleration of the erythrocyte blood sedimentation rate (BSR) in 86.1% of the patients (Table 2) . The following immunologic features were studied in patients with cardiac myxoma: complement activation; cellular activation; OKT4/8 ratio; serum levels of immunoglobulins, Ig subgroups, κ and λ chains of immunoglobulins, lymphocyte subpopulations, β-2 microglobulin (β-2 MG), interleukin 2R (Il-2R), intracellular adhesion molecule (ICAM), Fc epsilon receptor II (CD 23), tissue necrosis factor receptor (TNFR); anti-streptolysine titer (ASL), rheumatoid factor (RF); white blood cell count (WBCC); and serum protein electrophoresis (SPEP). Long-term survivors after resection of a myxoma still exhibited various immunologic anomalies (Table 3) . Furthermore, the immunologic status changed again in patients with recurrent disease (Fig. 1) . A familial occurrence of the disease was excluded in first-grade relatives of our patients.
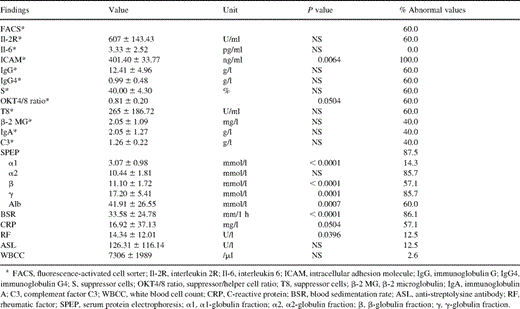
Preoperative immunologic alterations (n=49 patients, *n=5 patients)
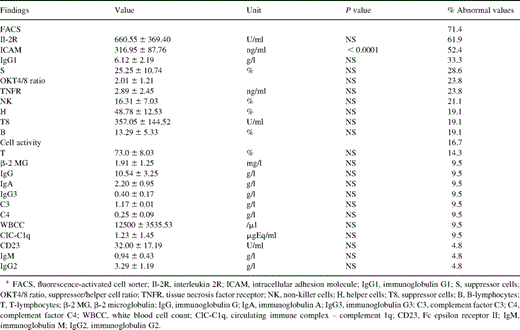
Immunologic alterations in long-term survivors after resection of a cardiac myxoma (n=21 patients)
2.2 Surgical technique
Surgical excision of the myxomas was performed under extracorporeal circulation and moderate hypothermia. Median sternotomy was used in all but two cases, one involving a right thoracotomy and the other a left thoracotomy. The surgical approach was uniatrial in 17 patients (34.7%). In 32 patients (65.3%) tumor resection was followed by inspection of both atria for more myxoma tissue. A combined bilateral atriotomy and transaortal resection of a mitral valve myxoma was performed in one case [6]. This myxoma arose in an extremely rare fashion from the ventricular side of the anterior mitral valve leaflet and nearly reached the supravalvular area of the ascending aorta [7]. A complete resection of the tumor together with an adjacent cuff of endocardium was performed, and either a direct suture (93.9%) or a patch closure (6.1%) of the defect followed. Nine patients were treated with combined procedures for additional coronary artery disease, mitral valve disease, or peripheral embolism. Macroscopically, the gelatinous tissue appeared as yellow-brownish to yellow-greenish myxoid stroma, which is typical for this tumor. Also, the stroma is thought to represent multipotent cells which are capable of many types of mesenchymal or epithelial differentiation [8]. Tumor size ranged between 1.3 and 15 cm (mean 4.7 cm). Histopathologic examination revealed proliferations of capillaries, blood extravasations, and disseminated fibrin depositions. These findings were consistent with the diagnosis of a myxoma. Intraoperative echocardiography showed normal mitral valve function in all patients and trivial aortic insufficiency in one patient.
2.3 Statistical methods
Follow-up of the survivors was accomplished by means of questionnaires, telephone calls, clinical and laboratory examination, and TTE or TEE. For comparison of patients with and without embolization of the myxoma, a two-tailed Fisher's exact test or χ2 test were applied. Estimations of the risk for tumor embolization were performed using logistic regression. Alterations of immunologic parameters were analyzed using the one-sided unpaired Student's t-test. Causes of death were ascertained from the National Cause of Death Register. Survival rates were calculated using the Kaplan–Meier method, with significance levels of P<0.05. SAS® was used for computation.
3 Results
3.1 Risk for tumor embolization
Thirteen preoperative, one intraoperative and two postoperative events of tumor embolization occurred. Possible risk factors were examined in these 16 patients. In search for common characteristics of these patients, we found a stalk supported myxoma in ten patients (76.9%), an atrial tachyarrhythmia in six patients (46.2%), a myxoma involvement of mitral valve tissue in five patients (38.5%), and a prolapse through the mitral valve in four patients (30.8%). A combination of two factors was present in 12 patients (92.3%). Comparing patients with and without embolization of the myxoma, significant differences could only be identified regarding tumor consistency (P<0.004). Gelatinous, soft, lobated and disruptive tissue was found more often in the group with embolization than sturdy, solid and encapsulated tumors. In this group, a trend for a higher mortality (P<0.052) and for more atrial tachyarrhythmias (P<0.070) was revealed. No differences were observed concerning sex, number of myxomas, anticoagulation treatment, origin of the myxoma and tumor prolapse through a valve. Only patients presenting a cardiac rhythm other than sinus rhythm (P<0.026) or a myxoma originating from the left atrium (P<0.070) were identified as having a significantly increased pre- and postoperative risk for tumor embolization. These two features were able to predict 33 of 36 ‘no embolies’, but only four of 13 embolies (32.1%) correctly. Thus, the classification was correct in 75% of cases, but rather bad concerning risk estimation.
3.2 Mortality
The follow-up period after resection of a myxoma ranged between 6 days and 24 years. Four of the 49 myxoma patients died postoperatively. One patient died on day 6 after the operation due to low cardiac output and subsequent multiorgan failure. Another patient died 8 months postoperatively after having developed an apallic syndrome from intraoperative embolization of the myxoma. In two other patients cardiac insufficiency from coronary artery disease developed many years after myxoma resection. There has been no intra- or perioperative death due to myxoma during the past 24 years. Early mortality was 2.0% due to one patient dying 6 days after operation. Late mortality was 6.1% due to three patients dying 8–177 months postoperatively. The overall survival rate was 74% at 24 years.
3.3 Early postoperative results
Nonfatal early complications were found in 21 patients (42.9%) and are listed in Table 4 . Cardiac and respiratory complications represented typical conditions after open-heart surgery and included postcardiotomy syndrome, pericardial effusion, prolonged healing of the sternal wound, bradycardia necessitating pacemaker implantation, atrial flutter, atrial fibrillation, respiratory insufficiency, bronchopulmonary infection, and pneumothorax. One patient developed an ileus. Cerebral complications were found in three patients, one with infarction in the area supplied by the posterior cerebral artery, one with reversible hemiparese and the other with transient disorientation syndrome.

3.4 Late postoperative results
Thirty-nine long-term survivors were contacted at a median of 6 years after the operation (mean 77 months, range 9 months to 24 years). Thirty patients (81%) were entirely asymptomatic. Seven patients (19%) reported cardiac symptoms. Twelve patients had supraventricular arrhythmias. Four patients were treated with aspirin and seven with warfarin. No thromboembolic event or stroke was observed.
From TTE and TEE examinations, a recurrence of the myxoma was suspected in one patient 72 months after his first operation for a myxoma. At that time, 21 out of our 49 patients were at risk for a recurrence of the disease (Fig. 2) . The rate of recurrence of the myxoma was 2.0% after 24 years. A symptomatic patient, a 45-year-old man, had three small myxomas that were resected in an uneventful reoperation; he is without symptoms since. Thus, the rate of reoperation for a cardiac myxoma in our patients was 2.0%. The actuarial freedom from recurrence of the disease was 95% at 24 years of follow-up (Fig. 2). Other conditions found with TTE or TEE were: mild to moderate mitral insufficiency in three patients, prolapse of the posterior mitral valve leaflet in one patient, dilation of both the left atrium and ventricle in two patients, aortic insufficiency grade II in one patient, and a massive incompetence of the left ventricle in one patient (Table 5) . Because one myxoma recurred during a total observation period of 344 patient-years, the linearized recurrence rate was 0.29 per 100 patient-years.
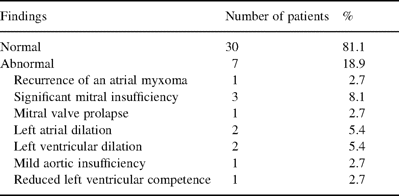
Echocardiographic findings in long-term survivors after resection of a cardiac myxoma (n=37 patients)
3.5 Immunologic parameters
Preoperative immunologic alterations were found in terms of BSR (86.1%), SPEP (87.5%), CRP (57.1%), and RF (12.5%) (Table 2). ICAM was tested in five patients and was elevated in all. The OKT4/8 ratio was reduced and suppressor cells (S) were slightly increased.
Out of 21 postoperative immunologic examinations, 15 patients (71.4%) presented abnormal values in terms of fluorescence-activated cell sorter (FACS) analysis, whereas five patients (23.8%) had normal values (Table 3). Abnormal ICAM values were found for 11 patients (52.4%).
In one case of a recurrent myxoma, immunologic alterations were examined and pre- and postoperative data were compared (Fig. 1). Preoperatively, serum CRP was elevated. FACS analysis revealed an impressive modification of the cell composition with an increased number of suppressor cells and thus a decreased helper/suppressor cell ratio of 0.8. C3 was elevated, while Il-2R, T8, and IgA were lower than normal. Immediately postoperatively, most immunologic parameters were altered due to the use of extracorporeal circulation. After 1 month, however, CRP had decreased to normal levels and the helper/suppressor cell ratio had normalized. C3, Il-2R, and T8 were normal, while IgA was borderline. Il-6 levels were normal.
4 Discussion
Although primary tumors of the heart are rare, the myxoma is the most frequent benign primary heart tumor as it accounts for 0.3% of open-heart surgery performed in our group and other groups worldwide [2,10]. The clinical presentation in the majority of patients consisted of important hemodynamic symptoms related to blood flow obstruction and embolic phenomena. Furthermore, the myxoma may threaten valve obstruction and, with future tumor growth, a left ventricular outflow tract obstruction. These patients can have an increased risk for acute cardiogenic shock or sudden cardiac death [9,11].
A higher risk of embolization has also been reported and events occur in 30–43% of the patients [2,3]. Embolization from a mitral valve myxoma might occur more often than from an atrial myxoma due to motion of the valve leaflets. In addition, the high pressure within the left ventricle during systole seems to give rise to embolizations more frequently from the left than from the right side of the heart [3]. Embolizations occur more often from polypoid tumors floating in the blood stream than from solid round tumors [12]. Tumor size may play an additional role in embolization [13]. We report in this study that patients presenting two certain characteristics were identified as having a significantly increased pre- and postoperative risk for tumor embolization.
Asymptomatic cases were rare. This important fact may have an impact on screening methods for such patients. Our data seem to indicate that screening for myxoma is important in those cases of familial occurrence, in complex myxoma, or Carney disease in particular [14]. In myxoma families, additional immunologic and genetic screening can identify family members at risk for the disease or for a possible recurrence of disease.
Significant immunological alterations in myxoma patients seem to occur pre- and postoperatively for BSR, SPEP, CRP, FACS, Il-2R, and ICAM (Tables 2 and 3). After surgical removal of the myxoma they return to normal. In cases of recurrence of the disease, these parameters may undergo a change again. We found alterations of ICAM, but not of the OKT4/8 ratio or S, also in up to 66.6% of the long-term survivors after resection of a cardiac myxoma without recurrence of the disease. The preoperative presence and the postoperative diminution of antibodies to fresh heart muscle has been demonstrated in some myxoma patients. Therefore, it is speculated that patients may have an immune response reaction to the neoplasm or to the heart muscle mediated by the presence of neoplasm, and the reaction leads to constitutional symptoms [15]. Immunological changes due to medication, intercurrent diseases, high age, and the use of the heart–lung machine can interfere with immunological screening for myxomas and should therefore be considered when interpreting immunological results. The patient from whom the data for Fig. 1 were derived was a 41-year-old male, who received none of these drugs around the pre- and postoperative examination days. When the 21 long-term patients underwent the postoperative immunological examination, some were treated with drugs, which could have altered their immune system. However, their measured values did not significantly differ from those measured in patients, who did not receive potentially immunomodulating drugs.
Differential diagnoses include thrombotic lesions, other cardiac tumors, and also but rarely neoplastic invasion of the inferior caval vein, for example, by a pheochromocytoma. MRI, in addition, allows the determination of the tissue density and may be used to distinguish myxomas from calcified and fatty tumors. Recently, calretinin, a new immunohistochemical marker, has been investigated and found to be highly specific in differentiating cardiac myxoma and mural myxoid thrombi [16]. Echocardiography, especially two- or three-dimensional TEE [12,17], is useful for estimating the risk for tumor embolization. Specifically, the risk seems to depend upon the shape, morphology, and mobility of the myxoma. Furthermore, both methods seem very helpful in planning the access for operation on valve tumors.
The surgical access to the myxoma may vary depending on the tumor location. Myxoma excision by way of aortotomy may be feasible in most cases. This approach facilitates the exposure of the left-ventricular-sided aspect of the mitral valve apparatus. In all the cases presented here, and in most cases presented in the literature, the originally compromised mitral, tricuspid, or aortic valves and the interventricular septum were completely preserved, and the patients were treated by resection of the tumor alone. Closure of an atrial septal defect (ASD) due to tumor resection was performed in one case. In those rare instances in which the tumor arises from an atrioventricular valve (AV) valve, the valve occasionally requires valvuloplasty or even replacement. Special care must be taken to avoid intraoperative systemic or pulmonary embolization of the myxoma. With systolic prolapse of some tumors into the left ventricular outflow tract, patients are at a higher risk of intraoperative embolization. For these reasons we decided to remove the tumor through the aortic root. A transventricular or minimally invasive approach, using the Heart-Port system, can be used as an alternative [18,19].
The prognosis for patients with solitary myxomas after surgical resection has been excellent. Postoperative complications were comparable to other cardiac operations (Table 4). Usually, the hospital mortality after the removal of an atrial myxoma is about 4% [2]. All current surgical techniques seem to provide low recurrence rates. Late recurrences have been reported to occur in 0.4–5% of surgically treated patients from up to 22 years after operation [2]. However, 40% of patients with familial myxoma experience recurrence of the myxoma [7]. Cardiac myxomas seem to recur more often in young males, or in patients with multifocal origins, and in those who have a family history of the tumor [2,7]. Therefore, it is necessary to perform routine echocardiography frequently throughout a patient's life. Except for patients with multifocal, atypical, or familial myxomas, echocardiography at 5-year intervals for several years should be adequate.
The TEE follow-up at 12 months was uneventful in 81% of our patients (Table 5). Trivial (grade I) mitral valve dysfunction without evidence of tumor recurrence was the most common abnormal finding in 7% of the patients. As the recurrence rate is higher in patients who already had a relapse, these patients demand special attention at follow-ups.
The courses of our patients indicate that the many risks inherent in this disease may be kept very low with immediate surgical treatment after early diagnosis with a particular focus on familial occurrence. As this disease may mimic a huge variety of other cardiac diseases, a possible myxoma should always be considered since relatively low risk cardiac surgical treatment is performable for most patients, and its postoperative prognosis is excellent.
References
- myxoma
- heart neoplasms
- left ventricular outflow obstruction
- mitral valve stenosis
- echocardiography
- sudden cardiac death
- left atrium
- right atrium
- atrial myxoma
- mitral valve
- atrium
- left ventricle
- embolization
- preoperative care
- repeat surgery
- surgical procedures, operative
- survival rate
- diagnosis
- heart
- mortality
- neoplasms
- embolism
- immunology
- early diagnosis
- atriotomy
- aortotomy




