-
PDF
- Split View
-
Views
-
Cite
Cite
Masazumi Watanabe, Koso Egi, Masato Shimizu, Hideki Nakahara, Hiroyuki Tanaka, Tohru Sakamoto, Makoto Sunamori, Non-depolarizing cardioplegia activates Ca2+-ATPase in sarcoplasmic reticulum after reperfusion, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 6, December 2002, Pages 951–956, https://doi.org/10.1016/S1010-7940(02)00582-1
Close - Share Icon Share
Abstract
Objectives: Depolarizing cardioplegia is the most common method for myocardial preservation in cardiac operations. However, depolarizing cardioplegia causes depolarization of the membrane potential by extracellular hyperkalemia, resulting in depletion of energy stores and calcium overload. This study examined the hypothesis that non-depolarizing cardioplegia would provide superior protection compared with depolarizing cardioplegia. Methods: In an isolated rat heart Langendorff model, hearts were perfused for 10 min with St. Thomas' Hospital cardioplegic solution (Group I: n=20), St. Thomas' Hospital cardioplegic solution+Lidocaine 1 mM (Group II: n=20) or non-depolarizing cardioplegia (Group III: n=20). The hearts then were subjected to 60 min of normothermic global ischemia, after which they were perfused with Krebs–Henseleit buffer at 37 °C for 30 min. The percent recovery of functional data, myocardial cyclic AMP contents, and myocardial cyclic GMP contents were recorded at each time point (base, after the administration of cardioplegia, after global ischemia, and after 30 min of reperfusion). Ca2+-ATPase in sarcoplasmic reticulum was measured at pre-ischemia and 30 min of reperfusion. Results: The percent recovery of developed pressure and ±dp/dt were significantly higher in Group III than in other groups. Myocardial cyclic AMP and GMP contents were elevated after reperfusion in all groups. However, in Group III, myocardial cyclic AMP contents after 30 min of reperfusion were significantly higher than in other groups (Group III: 14.7±1.6 vs. Group I: 8.7±1.0, Group II: 8.3±0.2 pmol/mg dry weight, P=0.05) but not cGMP. The sarcoplasmic reticulum Ca2+-ATPase activities at 30 min of reperfusion significantly increased in Group III compared with Groups II and I (Group III: 70.3±3.6 vs. Group I: 46.8±3.4, Group II: 53.9±6.1 μmol Pi/mg per h, P=0.025 and P=0.030). Conclusions: Non-depolarizing cardioplegia induced the activity of Ca2+-ATPase in sarcoplasmic reticulum after reperfusion. The activity would be increased by the cyclic AMP pathway. These findings suggested that non-depolarizing cardioplegia prevented calcium overload after reperfusion, especially decreased cytosolic calcium during the diastolic phase.
1 Introduction
Depolarizing cardioplegia is the most common method for myocardial preservation in cardiac operations. Potassium at high concentrations is the major depolarizing agent in cardioplegic solutions. At present, St. Thomas' Hospital cardioplegic solution (ST solution) is one of the most widely used solutions. This ST solution is a hyperkalemic solution that contains 16 mmol/l magnesium (Mg) and 1.2 mmol/l calcium (Ca). However, a recognized adverse effect of hyperkalemic cardioplegia is the possible development of ventricular dysfunction believed to be related, in part, to intracellular Ca2+ loading, a consequence of K+-induced membrane depolarization [1]. Despite various modifications implemented in hyperkalemic cardioplegic solutions, ventricular dysfunction is common and still contributes to the morbidity and mortality associated with cardiac surgery [2–4]. Therefore, we have used a non-depolarizing cardioplegic solution from 1994 and obtained good results in clinical experiences. However, the mechanism of beneficial effects is still unclear. A previous report has demonstrated that non-depolarizing solution retards myocardial calcium accumulation during cardioplegia [5]. Therefore, we hypothesize that non-depolarizing cardioplegia prevented Ca2+ overloading after reperfusion through the sarcoplasmic reticulum Ca2+-ATPase.
The present study was designed to compare the effect of non-depolarizing cardioplegia and the effect of depolarizing cardioplegia.
2 Materials and methods
The investigation was performed in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No 85-23, revised 1985).
2.1 Experimental preparation
Male Sprague–Dawley rats (body weight 250–300 g) were anesthetized by an intraperitoneal injection of pentobarbital (80 mg/kg) and heparinized (200 units via the femoral vein). Following thoracotomy, their hearts were rapidly excised and arrested in cold-modified Krebs–Henseleit bicarbonate buffer solution (mKHB buffer: NaCl 118 mEq/l, KCl 4.7 mEq/l, KHPO4 1.2 mEq/l, NaHCO3 24 mEq/l, MgSO4 1.2 mEq/l, glucose 10 mEq/l, CaCl2 1.7 mEq/l, gassed with 95% oxygen and 5% CO2, pH 7.4). The ascending aorta was cannulated and hearts were perfused in a retrograde fashion in the non-recirculating Langendorff mode with mKHB buffer at 37 °C at a pressure of 100 cmH2O for 10 min. Myocardial temperature was maintained at 37 °C by a surrounding water jacket.
2.2 Experimental protocol
The experimental protocol is represented as a time line in Fig. 1 . After an initial 10 min perfusion, hearts were perfused for another 10 min with ST solution (NaCl 110, KCl 16.0, MgCl2 16.0, CaCl2 1.2, NaHCO3 10.0 mmol/l; Group I, n=20), or with ST solution containing 1 mM Lidocaine (Group II, n=20) or with non-depolarizing cardioplegic solution (NaCl 110, KCl 5, MgCl2 10, CaCl2 5.4, NaHCO3 10 mmol/l; lidocaine, 1 mM/l; Group III, n=20). The hearts then were subjected to 60 min of normothermic global ischemia, after which they were perfused with mKHB buffer at 37 °C for 30 min. Contractile functions were obtained at baseline before the first perfusion, and after 30 min of reperfusion. The membrane resting potential in Purkinje fibers was as follows: −71 mV (2 min) and −52 mV (60 min) [5].
Experimental protocol. A timeline is given for the study as conducted for each group. After 10 min of Langendorff perfusion (L-P) with normal modified Krebs–Henseleit bicarbonate buffer (KHB), baseline myocardial function was analyzed. Following this, cardioplegic solution was perfused for a total of 10 min. After 30 min of global ischemia, the heart was reperfused with normal KHB for 30 min. Myocardial function was measured and reassessed after reperfusion. Biopsies were taken for cAMP, cGMP and Ca ATPase measurements before ischemia and after 30 min and reperfusion in separate hearts. (I) St. Thomas' Hospital cardioplegic solution; (II) St. Thomas' Hospital cardioplegic solution+Lidocaine 1 mM; (III) non-depolarizing cardioplegia.
2.3 Myocardial contractile function
Aspects of left ventricular function evaluated included heart rate (HR, beats/min), maximum developed pressure (max DP, mmHg), the first derivative of the left ventricular pressure (LV±dp/dt, mmHg/s), and coronary blood flow. Pressures were measured with a fluid contained compliant balloon (0.5 ml), which was connected by fluid-filled polyethlene tubing to a pressure transducer (P23ID; Gould, Inc., Cleveland, OH). The balloon was inserted into the left ventricle through the mitral valve and diastolic pressure was set at 5–10 mmHg. DP was measured as the difference between peak systolic pressure and end-diastolic pressures. Coronary flow (CF; ml/min) was assessed by measuring the volume of coronary effluent.
2.4 Measurement of myocardial cyclic AMP and GMP contents
After each experimental point (each n=6), the left ventricles were quickly removed and immediately frozen in liquid nitrogen. The homogenate was centrifuged at 10 000×g. Trichloracetic acid (5%) in the supernatant was removed by ether extraction. After reconstitution in assay buffer, samples were assayed for cAMP and cGMP by using a commercially available Biotrak Enzyme-linked Immunosorbent assay kit from Amersham Corporation (Arlington Heights, IL). The assay is based on competition between unlabeled cAMP (cGMP) and a fixed quantity of peroxidase-labeled cAMP (cGMP), for a limited number of binding sites on a cAMP (cGMP) specific antibody.
2.5 Measurement of Ca2+-ATPase activity
Sarcoplasmic reticulum membrane vesicle fractions were isolated from cardiac microsomes. Cardiac sarcoplasmic reticulum was purified as described by Jones et al. [6]. A microassay for ATPase was determined using the method described by Chan et al. [7,8]. This method used the spectrophotometric measurement of inorganic phosphate released from ATP (Pi). In this assay, 0.5 ml of samples or standards was mixed with 2 ml of malachite green reagent (Sigma). After the malachite green reagent was added to samples and standards, the relative absorbance of the samples compared to a reagent blank was measured at 630 nm using the spectrophotometer.
2.6 Statistical analyses
Statistical analysis was performed using a Macintosh computer (8100) with Statview IV statistical software (Abacus Concepts, Berkeley, CA). All values are expressed as mean±standard error. Data were analyzed using repeated measures analysis of variance and Dunnett's t-test where appropriate. P values less than 0.05 were considered statistically significant.
3 Results
3.1 Hemodynamics
No episodes of ventricular arrhythmia occurred after reperfusion in either group. LV functional recovery data for the three groups at baseline before entering the group protocols and after 30 min of reperfusion are displayed in Table 1 . In Group III, DP and ±dp/dt were significantly higher than in the other groups after 30 min of reperfusion (DP: Group III vs. II and I, P=0.012 and P=0.014; +dp/dt: Group III vs. II and I, P=0.015 and P=0.017; −dp/dt: Group III vs. II and I, P=0.021 and P=0.033). HR and CF rate were significantly decreased after reperfusion in all groups compared with pre-ischemic data (P=0.0005). However, no significant differences in HR and CF rate were noted among all groups.
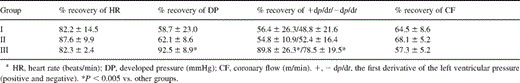
3.2 Myocardial cyclic AMP and cyclic GMP content (Fig. 2)
Myocardial cyclic nucleotides contents. Myocardial cyclic nucleotide concentrations (mean±SEM) obtained before ischemia (Base), after cardioplegia infusion (plegia), at the end of ischemia (after ischemia), at 30 min of reperfusion (R30). *P<0.05 vs. before ischemia, cAMP (Base vs. plegia and R30, Group I: P=0.005 and P=0.006, Group II: P=0.001 and P=0.0001, Group III: P=0.021 and P=0.002), cGMP (Base vs. plegia and R30, Group I: P=0.0004 and P=0.001, Group II: P=0.001 and P=0.001, Group III: P=0.031 and P=0.009). #P<0.05 vs. Groups II and I (P=0.028 and P=0.013). Left graph: the changes in cyclic AMP; right graph: the changes in cyclic GMP.
The changes of cAMP and cGMP contents in the experiment are shown in Fig. 2. After 10 min of cardioplegia infusion, cAMP and cGMP content were significantly increased in all groups compared with baseline levels (cAMP [cGMP]; Group I: 10.17±1.22 [1.84±0.13], Group II: 7.15±0.41 [1.91±0.20], Group III: 9.29±1.48 [1.37±0.30] vs. baseline: 3.07±0.41 [0.23±0.13] pmol/mg dry weight, P<0.05). At the end of global ischemia, cAMP contents in all groups were recovered to baseline levels. After 30 min of reperfusion, cAMP and cGMP contents in all groups were significantly increased and cAMP content in Group III was significantly higher than in other groups (Group I: 8.71±0.98, Group II: 8.27±0.17 vs. Group III: 14.68±1.64 pmol/mg dry weight, P=0.013 and P=0.028). However, there were no significant differences in cGMP content among the groups.
3.3 Ca2+-ATPase activity (Fig. 3)
Ca ATPase activity. The Ca ATPase activity of sarcoplasmic reticulum (mean±SEM) obtained before ischemia (Base), at 30 min of reperfusion (R30). *P=0.01 vs. before ischemia, #P<0.05 vs. other groups (vs. Group I, P=0.025 and vs. Group III, P=0.03).
In Group III, myocardial Ca2+-ATPase activity significantly increased after 30 min of reperfusion compared with the pre-ischemic value (pre-ischemia: 48.7±4.87 vs. Group III: 70.3±3.59 μmol Pi/mg per h, P=0.01). In Groups II and I, however, there were no significant differences compared with the pre-ischemic value (Group I: 46.8±3.36, Group II: 53.9±6.10 vs. pre-ischemia, NS). In Group III, Ca2+-ATPase activity was significantly higher than in other groups (Group III vs. Group I, P=0.025 and Group III vs. Group II, P=0.03, n=8 in each group).
4 Discussion
Hyperkalemic cardioplegic solutions effectively arrest the heart during cardiac operations by depolarizing the sarcolemma [9,10]. Depolarization of the membrane is associated with an ongoing cellular metabolic process and derangements in transmembrane ionic gradients. It is also associated with an influx of sodium through the sodium ‘window current’, exchange of intracellular sodium for calcium via the sodium–calcium exchange, influx of calcium through the calcium ‘window current’, and leakage of calcium from the sarcoplasmic reticulum during the arrest. While Ca2+ is important in cell metabolism as a second messenger, extreme accumulations in the cytosol cause cell injury. Such accumulation in hearts during ischemia-reperfusion is known as Ca2+ overload, which contributes to reperfusion injury including myocardial stunning after cardioplegic arrest [11,12]. Ca2+ overload is caused by failure of three regulatory systems of cytosolic Ca2+ content: the cell membrane, through which Ca2+ normally cannot pass; Ca2+ pumps and channels related to the membrane; and mechanisms sequestering intracellular Ca2+ stores. Previous investigators have found that inhibition of Ca2+ overload with various calcium channel blockers reduces reperfusion injury. The sarcoplasmic reticulum is also critical to regulation of intracellular Ca2+ stores. Myocardial contractility depends on Ca2+ release from and uptake into the sarcoplasmic reticulum. The Ca2+ gradient between the sarcoplasmic reticulum matrix and the cytosol (sarcoplasmic reticulum Ca2+ gradient) is maintained by the sarcoplasmic reticulum Ca2+-ATPase using the free energy available from hydrolysis of ATP. The activity of the sarcoplasmic reticulum Ca2+-ATPase is not only dependent on the energy state of the cell but is also kinetically regulated by sarcoplasmic reticulum proteins such as phospholamban [13]. In steady state, the Ca content of the sarcoplasmic reticulum of cardiac myocytes is determined by a balance among influx and efflux pathways.
The sarcoplasmic reticulum Ca content may be limited mainly by the ATP-supplied chemical potential that is inherent in the gradient between sarcoplasmic reticulum and cytosol. The disruption of sarcoplasmic reticulum function represents a severe stress to cells. A reduction in sarcoplasmic reticulum Ca2+-ATPase activity contributes to the impairment in both systolic and diastolic function of failing human hearts [14–16]. And activated Ca2+-ATPase in sarcoplasmic reticulum reduces calcium overload during ischemia [17,18]. Therefore, the present study tested the hypothesis that non-depolarizing cardioplegia would activate sarcoplasmic reticulum Ca2+-ATPase in ischemia-reperfused myocardium and improve Ca overload and contractile function.
The major finding of the present study is that non-depolarizing cardioplegia prevented Ca2+-ATPase activity in sarcoplasmic reticulum after ischemia. Previous investigators have reported that Ca2+-ATPase activity was decreased in ischemia and increased in reperfusion [19]. In the present study, we showed that sarcoplasmic reticulum ATPase returned to the pre-ischemic values after reperfusion in depolarizing cardioplegia groups. However, in the non-depolarizing cardioplegia group, sarcoplasmic reticulum Ca2+-ATPase activity significantly increased at the 30 min of reperfusion period compared with other groups. These results indicate that calcium overload may be prevented in the non-depolarizing cardioplegia group compared with the depolarizing cardioplegia group.
Another interesting finding is the increase in myocardial cyclic AMP contents during cardioplegic infusion and ischemia/reperfusion. We showed the elevation of cAMP levels in rat myocardium after depolarizing cardioplegic arrest and non-depolarizing cardioplegia (P<0.05 vs. baseline). And the levels in the non-depolarizing cardioplegia after reperfusion were significantly higher than in depolarizing cardioplegia. Cooper et al. [20] have demonstrated in cerebellar granule cells that adenylyl cyclase can be regulated by membrane depolarization and, as a result of membrane depolarization, the influx of Na+, as well as Ca2+, will elevate cAMP activity [21]. Sunamori et al. [5] demonstrated that non-depolarizing cardioplegia prevented depletion of myocardial cAMP concentration. Although the mechanism is unclear, non-depolarizing cardioplegia also elevates cAMP activity in the present study. Moreover, at the reperfusion period, our data suggested that non-depolarizing cardioplegia might activate adenylyl cyclase through some pathways.
Previous studies have reported on cAMP and sarcoplasmic reticulum Ca2+-ATPase [22–24]. Ozawa [23] demonstrated that cAMP activated a ryanodine-sensitive Ca2+ release mechanism in the endoplasmic reticulum and that this activation is via a PKA-dependent process. Therefore, it is possible that the elevation of cAMP in reperfusion will activate sarcoplasmic reticulum Ca2+-ATPase and prevent Ca overload. Their findings support the possibility. It has been reported that cyclic GMP depressed Ca2+ mobilization by improving Ca2+-ATPase activity by phosphorylation [25]. However, Ishii et al. [26] demonstrated that cGMP inhibitor did not block the activity of sarcoplasmic reticulum Ca2+-ATPase. Our results showed that cGMP activity also increased after reperfusion compared to the pre-ischemic value. However, there were no significant differences among the groups. Therefore, we suggest that cAMP activity is associated with sarcoplasmic reticulum Ca2+-ATPase.
In summary, the results of this study indicate that non-depolarizing cardioplegia prevents ischemia/reperfusion injury via sarcoplasmic reticulum Ca2+-ATPase. And its protective effect of non-depolarizing cardioplegia is better than depolarizing cardioplegia. These data suggest the utility of non-depolarizing cardioplegia in clinical use. However, the present study is not designed to test in the surgical situation. Therefore, these results and effects may be limited to this model.




