-
PDF
- Split View
-
Views
-
Cite
Cite
Göran Dellgren, M.J. Eriksson, L.å. Brodin, K. Rådegran, Eleven years’ experience with the Biocor stentless aortic bioprosthesis: clinical and hemodynamic follow-up with long-term relative survival rate, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 6, December 2002, Pages 912–921, https://doi.org/10.1016/S1010-7940(02)00584-5
Close - Share Icon Share
Abstract
Objective. The long-term durability and hemodynamics of stentless valves are largely unknown. Our aim was to prospectively investigate long-term hemodynamic function and clinical outcome after aortic valve replacement with the Biocor stentless aortic bioprosthesis. Patients and methods. Between October 1990 and November 2000 we inserted the Biocor stentless aortic valve in 112 patients (male/female: 38:74) with a mean age of 78.5 years (median 79.3, range 60–88). The predominant diagnosis was aortic stenosis in 86% of the patients. Concomitant coronary artery bypass surgery was performed in 31% of the patients. Average prosthetic valve size was 23.3±1.6 mm. All patients were followed in a prospective study with a mean follow-up of 66±33 months. The follow-up was 100% complete with a closing interval from October 1 to December 31, 2001. The observed actuarial survival of patients was compared to expected survival for an age- and gender-matched comparison population as calculated from Swedish life tables by Statistics Sweden. Relative survival rates were calculated annually for the patient population. Results. Early mortality was 7% (8/112). Late mortality was 38% (43/112). Actuarial survival at 5 and 9 years was 74±5% and 38±7%, respectively. Observed survival among patients was not different from the expected survival for the comparison population and calculation of relative survival rates indicates a ‘normalized’ survival pattern for the patient population. At 5 and 9 years the actuarial freedom from valve-related death was 94±3% and 86±6%; from cardiac death, 82±4% and 57±8%; from valve reoperation, 96±2% and 87±6%; from structural valve degeneration, 96±2% and 87±6%; from thromboembolism, 89±4% and 71±9%; and from endocarditis, 96±2% and 90±5%. At 9 years the transvalvular mean pressure difference for all valves was 7.3±1.3 mmHg (range 6–10 mmHg) measured with Doppler echocardiography. Aortic regurgitation progressed slowly over time in a few patients and necessitated reoperation in two patients. Conclusion. The Biocor stentless bioprosthesis has an excellent hemodynamic function and confers a good long-term outcome. This patient population could be regarded as ‘cured’ from valve disease since the observed survival did not differ from the expected survival for an age- and gender-matched Swedish comparison population, a conclusion that is also supported by a constant relative survival after the first postoperative year. However, despite excellent long-term hemodynamics, patients with stentless bioprostheses need to be evaluated with echocardiography at regular intervals to discover the rare cases of progressive aortic regurgitation.
1 Introduction
The use of stentless porcine xenografts was originally described by Binet et al. in 1965 [1] but reintroduced, both experimentally and clinically, in the modern era by David et al. in 1988 [2,3]. Stentless aortic valve bioprostheses have demonstrated hemodynamic superiority over stented valves in a number of studies [4–8]. Two retrospective case-control studies also showed that patients who received a stentless valve survived longer than those who received a stented bioprosthesis [9,10]. This study reports the long-term results of the use of the Biocor stentless bioprosthesis in an elderly population.
2 Material and methods
2.1 Aortic valve prosthesis
The Biocor stentless (BS) aortic valve prosthesis (Biocor Industria e Pesquisas Ltda., Belo Horizonte, Brazil; from September 1996, St. Jude Medical, St. Paul, MN, USA) consists of three selected porcine aortic valve cusps mounted in a ring of bovine pericardium. The ‘Extended’ Biocor stentless (EBS) bioprosthesis is a BS bioprosthesis with added pericardial extensions, extending both superiorly (‘collar’) and inferiorly (‘skirt’) from the bovine pericardial ring and has previously been described [11]. The BS and the EBS bioprostheses are thus similar except for the pericardial extensions. The EBS bioprosthesis provides the option of enlarging the aortic root down to or into the mitral valve as well as up into the aortotomy. When not needed the extensions can be cut off and the valve used as a regular stentless valve.
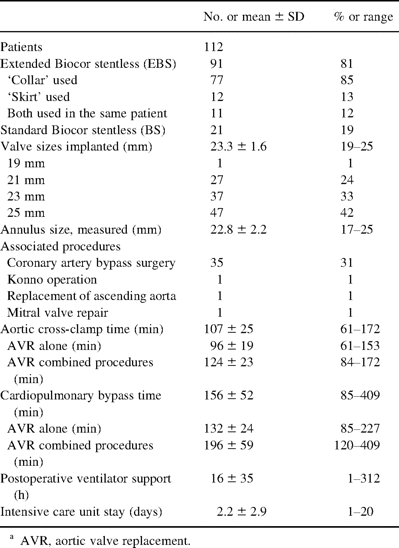
2.2 Patients
Between October 1990 and November 2000, 112 patients (median age 79.3 years) underwent 113 procedures with aortic valve replacement (AVR) with either a BS or an EBS bioprosthesis all performed by one surgeon (KR) at the Karolinska Hospital. Patient selection criteria were primarily symptomatic aortic valve disease and age >70 years. Detailed patient data are shown in Table 1 . We deliberately sought to include patients with a narrow aortic root, which explains the high percentage of older women. All patients gave their informed consent to participate in a prospective study, approved by the Ethics Committee at the Karolinska Hospital. Patients were seen annually for clinical examination and echocardiography. Those not able to come were contacted by phone. The closing interval for this study was between October 1 and December 31, 2001. The mean follow-up was 66±33 months and was 100% complete. The total follow-up was 562 patient years.

2.3 Operative technique
Midline sternotomy and cardiopulmonary bypass were used in all patients. After aortic cross-clamping, retrograde cold crystalloid (12/112) or blood (100/112) cardioplegia was administered through a coronary sinus catheter. Table 2 shows the operative data. An oblique aortotomy into the noncoronary sinus was used in most of the patients for the EBS valve. The incision was elongated down to or into the aortic-mitral curtain if the aortic root was considered very narrow. A transverse aortotomy was usually used for the BS valve. After excision of the aortic valve, the annulus was measured with the Biocor sizers. The selected prosthesis was implanted into the aortic root with a technique similar to the ‘freehand’ technique used in allograft surgery. When deemed desirable the lower pericardial extension was used to widen the aortic annulus and the upper extension was patched into the aortotomy. The proximal valve suture line was performed with either isolated 4-0 braided polyester sutures or running 3-0 polypropylene sutures. The distal suture line was undertaken with 4-0 polypropylene sutures, starting under the right and left coronary ostiae.
2.4 Doppler echocardiography
Transthoracic echocardiography with continuous-wave (CW) Doppler, pulsed-wave (PW) Doppler and color flow Doppler studies were performed using an Acuson 128XP (Acuson, Mountain View, CA, USA) equipped with a 2 MHz transducer. The maximum systolic blood velocity (Vmax) across the prosthesis was recorded. The average of three consecutive cardiac cycles in sinus rhythm or of five cardiac cycles in atrial fibrillation was used to calculate Vmax and velocity time integral. Maximum and mean pressure differences were calculated using the simplified short form of the Bernoulli equation [12]. Heart rate, blood velocity and velocity time integral in the left ventricular outflow tract were measured. The outflow tract diameter was used to calculate a circular cross-sectional area of the left ventricular outflow tract. The effective orifice area (EOA) and cardiac output were calculated with the continuity equation [13]. Aortic regurgitation (AR) was assessed using color flow Doppler, CW and PW Doppler, and classified as absent (0), trivial (+1), mild (+2), moderate (+3) or severe (+4).
2.5 Definitions and data analysis
This report adheres to the guidelines for reporting morbidity and mortality after cardiac valvular operations [14]. Early mortality is defined as hospital mortality, which is death within any time interval after the operation if the patient has not been discharged from the hospital. The continuous variables are reported as mean±SD. Differences of the means were tested for statistical significance with one-way analysis of variance (ANOVA) for repeated measurements. When the F-test revealed a significant difference, each pair of means was compared in Scheffe's test. The null hypothesis was rejected when a P-value was less than 0.05 and was considered statistically significant. ‘Linearized’ incidence was used to summarize the incidence of thromboembolic events since a few patients had multiple events [15]. Multivariable regression analysis performed according to Cox proportional hazard model (backwards selection) was used on the variables in Table 1 to analyze risk factors for late death [16]. The multivariable analyses were performed in SAS (version 8.0, SAS Institute Inc., Cary, NC, USA).
2.6 Observed and expected survival
Observed survival for patients was analyzed using life table technique and Kaplan–Meier estimates [17]. The expected survival was calculated in collaboration with Statistics Sweden (the Swedish population bureau) in an ‘exact’ way from Swedish life tables with a specially designed software program [18]. An assigned comparison group, consisting of all Swedish inhabitants of the same sex and age who were alive at the time (same month) of operation, was individually constructed for every patient. From these individually based expected survival curves a composite survival curve was constructed and subsequently compared to the observed survival curve of the patients. Age heterogeneity was deemed not to be an issue in this patient cohort and therefore the successive rejuvenation process was not adjusted for. The expected survival is based on calculations from the entire Swedish population and therefore errors of sampling do not apply and no standard errors are provided. One of the illustrations (Fig. 2) is graphically presented with a logarithmic y-axis because this facilitates a correct visual comparison between the two different survival curves [19]. In this setting, subgroups with equal hazard rates during a specified time interval have parallel survival curves. Life table analyses and Kaplan–Meier curves were constructed with the computer program Statistica (version 5.5).
2.7 Calculation, presentation and interpretation of relative survival rates
Relative survival rates have previously been used to describe long-term survival after heart valve replacement [19]. We have calculated the relative survival rates only taking yearly intervals into consideration with an annual adjustment of life tables, which start at the time of operation. A normalized survival pattern for the study group is represented by a constant relative survival from that time on. Therefore, the fraction of surviving patients has only the normal risk of dying and could be considered ‘cured’ from a statistical point of view. When the annual relative survival rate stabilizes around 1.0 the fraction of surviving patients will represent the ‘cured’ fraction. In contrast, an increased risk of death in the study group would be represented by a continuously decreasing relative annual survival rate.
3 Results
3.1 Patient survival
There were eight early (7%) and 43 late deaths (38%). Four of eight (50%) early deaths and 20 of 43 (46%) late deaths occurred among patients who underwent a combined AVR and coronary artery bypass grafting (CABG) procedure. The early morbidity and mortality as well as causes of late mortality are shown in Table 3 . The actuarial survival for hospital survivors is shown in Fig. 1 . Multivariate analysis identified female gender (Hazard ratio 1.99 (confidence interval (CI) 1.03–3.83), P<0.039) and preoperative myocardial infarction (hazard ratio 4.24 (CI 1.63–11.0), P<0.003) as independent risk factors for late death.

Actuarial survival for hospital survivors operated with the Biocor bioprosthesis. Horizontal bars indicate 95% confidence interval.
3.2 Expected survival and relative survival
There was no significant difference between the survival of AVR patients (including early and late mortality) and the expected survival for the age- and gender-matched population supplied by Statistics Sweden (log rank P=0.58) (Fig. 2) . The annual relative survival rate indicates a normalized survival pattern for patients operated with the Biocor stentless bioprosthesis (Fig. 3) . During the first postoperative year there was a higher mortality among the study patients as indicated by the 95% confidence interval being below 1.0. Interestingly, after the first postoperative year patients seem to have a survival advantage for several years in relation to the comparison population. The relative survival rate drops significantly at 9 years of follow-up but at that point there are only a small number of patients at risk.
Actuarial survival for all patients (including early and late deaths) operated with the Biocor bioprosthesis (continuous line) and for the age- and gender-matched control group (dashed line) derived from Statistics Sweden. Horizontal bars indicate 95% confidence interval for patient population. The expected survival is based on calculations from the entire Swedish population and therefore no standard errors are provided. Graphically presented with a logarithmic y-axis because this facilitates visual comparison between different survival curves.
Annual relative survival rates for patients operated with the Biocor stentless bioprosthesis. Annual relative survival rates are calculated on yearly intervals as a ratio between survival for patients and for the age- and gender-matched Swedish comparison group. Horizontal bars indicating 95% confidence interval for relative survival.
3.3 Valve- and cardiac-related deaths
Causes of valve- and cardiac- related deaths are listed in Table 3. The actuarial freedom from valve-related death at 5 and 9 years was 94±3% and 86±6%, respectively (Fig. 4) . The actuarial freedom from cardiac death at 5 and 9 years was 82±4% and 57±8%, respectively (Fig. 4). The actuarial freedom from non-cardiac death at 5 and 9 years was 85±4% and 63±8%, respectively (Fig. 4).
The actuarial freedom from valve-, cardiac- and noncardiac-related deaths.
3.4 Late thromboembolism
Late thromboembolic events were observed in 12 patients (13 strokes, three transient ischemic attacks). The linearized incidence of thromboembolism was 2.8±0.7 events/100 patient years. The actuarial freedom from thromboembolism at 5 and 9 years was 89±4% and 71±9%, respectively. At their last follow-up, 12 (12/104, 11%) patients were taking warfarin sodium, 62 (62/104, 60%) patients were taking aspirin daily and 30 (29%) patients were not receiving anticoagulation or antiplatelet therapy. There was a significant difference in mortality in patients who where without anticoagulation- or antiplatelet-therapy at their last follow-up (18/30, 60%) compared to those treated with warfarin sodium (4/12, 33%) or aspirin (22/62, 35%) (P<0.05). Multivariate analysis performed on preoperative characteristics (Table 1) could not identify any independent risk factors for late thromboembolism.
3.5 Structural valve dysfunction
Structural valve deterioration occurred in two patients. Both patients had commissural tears without signs of calcification and were reoperated because of progressive AR after 3.9 and 7.8 years of follow-up. The actuarial freedom from structural valve dysfunction at 5 and 9 years was 96±2% and 87±6%, respectively.
3.6 Bioprosthetic valve endocarditis
One patient had early bioprosthetic valve endocarditis secondary to postoperative mediastinitis. This stentless valve was replaced with another EBS valve in the early postoperative period. No patient experienced late endocarditis. The actuarial freedom from bioprosthetic valve endocarditis at 5 and 9 years was 96±2% and 90±5%, respectively.
3.7 Reoperations
Three patients underwent reoperation. The indication for reoperation was structural valve deterioration in two patients and early endocarditis in one patient. All patients survived the reoperation uneventfully. The actuarial freedom from reoperation at 5 and 9 years was 96±2% and 87±6%, respectively.
3.8 Other complications
Even though 11% of the patients were receiving oral anticoagulation, only one patient experienced an anticoagulant-related hemorrhage (epistaxis), and this did not necessitate hospitalization or transfusion. Eight patients needed late pacemaker implantation. Two patients suffered a late acute myocardial infarction.
3.9 Late functional classification
At late follow-up (closing interval October 1–December 31, 2001, mean follow-up 5.5±2.8 years), 59 patients were alive and had their original bioprosthesis (51 were dead and two were reoperated). Thirty-nine patients (66%) were in New York Heart Association functional class I, 16 (27%) in class II, 4 (7%) in class III and none in class IV.
3.10 Echocardiography
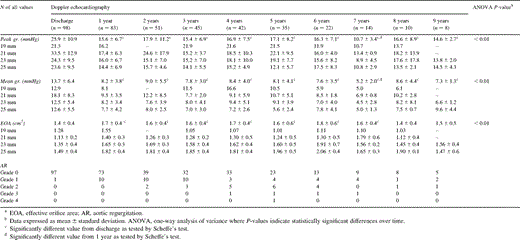
Peak and mean pressure differences calculated with the short form of the Bernoulli equation for all valve sizes were 14.6±2.7 mmHg (range 11–19 mmHg) and 7.3±1.3 mmHg (range 6–10 mmHg), respectively, at 9 years of follow-up (Table 4) . Both peak and mean systolic pressure differences were significantly lower at 1 year compared to those at discharge. There was no further significant change in these hemodynamic parameters over time. The EOA was for all valves 1.5±0.5 cm2 (range 1.0–2.1 cm2) at 9 years of follow-up. The EOA had increased significantly at 1 year compared to that at discharge. There was no further significant change in EOA over time. AR progressed slowly over time in a few patients and necessitated reoperation in two patients (Table 4).
Hemodynamic follow-up of Biocor stentless (EBS and BS) aortic valve bioprosthesis
4 Discussion
Late survival after aortic valve replacement with the Biocor stentless aortic bioprosthesis may appear low when compared to other series of stentless valves. These series consist, however, of considerably younger patients [3,9]. The majority of our patients died of cardiac- or noncardiac- related causes within the first 10 years after operation and only few died due to valve-related problems. Survival of this elderly patient population was also not significantly different from an age- and gender-matched Swedish control group supplied by Statistics Sweden. In fact, aortic valve replacement with the Biocor stentless valve seemed to give this elderly patient population a normalized survival as illustrated by a more or less constant relative survival from that time on. The lower relative survival rate during the first postoperative year seems mainly related to the early, in-hospital mortality. Interestingly, after the first postoperative year, these patients seem to survive better than the comparison population. However, this may be due to a selection bias, towards in general a healthy patient population with few concomitant diseases at the time of operation. Towards the end of the study period, at 9 years of follow-up and thereafter, the relative survival rate drops significantly, which is most likely related to the small number of patients at risk. These patients may thus be regarded as ‘cured’ from their valve disease even though approximately 30% of them had concomitant coronary artery disease. A study from our institution has previously shown that patients older than 65 years of age with pure aortic stenosis and operated with isolated mechanical valve replacement also achieved a normalized survival pattern [19]. In contrast to our study, however, that patient population included few very old patients and few had combined procedures performed. The long-term survival of our patient cohort is well in line with what Gehlot et al. reported for a similar population of elderly patients undergoing aortic valve replacement with a stented bioprosthesis [20]. Our study suggests that also in elderly patients, even those needing a combined procedure, the stentless valve can be used successfully.
Implantation of a stentless valve is a technically more complex procedure than implantation of a stented alternative. Nevertheless, the early mortality and morbidity in our series compares well with what has been reported for other series of stented valves in elderly patients [20,21]. Compared with younger age groups, aortic valve replacement in patients 80 years of age and older has been shown to be associated with a distinctly increased early mortality (14%) and morbidity [21]. Gehlot et al. reported an early mortality of 14% in a population similar to ours but with a somewhat higher mean age (82.7 years) [20]. Early mortality of 7% in our study is higher than what has been reported for the Toronto SPV valve by David et al. [3], but may be explained by a difference in mean age of 15 years (78.5 versus 63.3 years) between the series. Westaby et al. reported a similar early mortality of 8% for the Freestyle stentless valve in a consecutive, unselected but somewhat younger patient population (mean age 73.0 years) [9].
Others have shown in retrospectively matched studies that stentless valves might confer a survival advantage compared to stented bioprostheses [9,10]. However, so far no prospective randomized study has been published comparing stentless valves with other prostheses and therefore conclusions have to be cautious. If this survival advantage for stentless valves is real, it suggests a reduction of late valve related events as well as of late cardiac events compared to stented prostheses. Stentless valves have shown excellent both early and long-term hemodynamics in several other studies [4,6–8] as well as in our long-term follow-up of the Biocor bioprosthesis. Stentless aortic bioprostheses have, compared to the stented alternatives, a favorable hemodynamic profile, which is similar to that of the native aortic valve or the aortic allograft [5,8]. Transvalvular pressure gradients are lower and EOA is larger, and therefore left ventricular wall stress is less for stentless valves than for stented ones. The Biocor stentless valve had at 9 years of follow-up a mean transvalvular gradient of 7 mmHg, which is very similar to what has been reported for the Toronto SPV [6]. Moreover, the regression of transvalvular gradients and the increase of EOA within the first postoperative year reported for other stentless valves were also seen for the Biocor stentless valve [6–8].
Left ventricular hypertrophy (LVH) is associated with an increased risk of arrhythmias and sudden death, a common cause of death in the natural history of aortic stenosis [22]. LVH is very common in patients with aortic stenosis and more pronounced in patients with certain genotypes [23]. Reduction of LVH has been shown to be complete and remain constant for at least five years in patients operated with stentless valves [6]. This is likely related, at least in part, to low transvalvular gradients. However, we have previously also shown that the regression of left ventricular hypertrophy is multifactorial and may be influenced by genetics as well as gender, coronary artery disease, hypertension, aortic valve pathology and other yet unknown factors [6,23]. A normalization of the left ventricular mass has the potential to reduce long-term cardiac related events and cardiac deaths in patients operated with aortic valve replacement. Our present study showed that late valve-related events were few with the Biocor bioprosthesis. Two patients were reoperated on because of slowly progressing AR. David et al. reported this to occur only rarely for the Toronto SPV valve [24]. Moreover, there seem to be very few episodes of late endocarditis and anticoagulant related bleedings among stentless valve bearers, not only in our study but also as reported by others [25]. However, our survival rates were very similar to those of patients mostly receiving stented bioprostheses, as reported by Gehlot et al., suggesting no difference in cardiac-related events or deaths between stented and stentless valves [20]. Therefore, though the survival advantage for stentless valves seems logical considering the regression of LVH and few valve related events, this needs to be proven in a prospective randomized study.
4.1 Limitations of study
This study is a study of the Biocor stentless aortic bioprosthesis and therefore the likelihood of underestimating valve related events is limited to this specific prosthesis. The interpretations of our results have to be careful since there might be a bias in patient selection, even though we sought to include patients with small aortic root, as shown by the higher than average proportion of elderly women included in this study.
4.2 Conclusion
The Biocor stentless bioprosthesis showed excellent long-term hemodynamic function and was, considering the advanced age of the study population, associated with a good long-term outcome. Our patients could be regarded as ‘cured’ from their valve disease, as was clear when their survival was compared to that of the age- and gender-matched Swedish control population. Patients with a stentless bioprosthesis need to be evaluated at regular intervals with echocardiography to monitor progressive aortic regurgitation, although this develops only rarely. Finally, a prospective randomized study is necessary to determine whether the use of stentless bioprostheses is associated with a survival advantage.
Karolinska Institutet, the Swedish Heart and Lung Foundation and the Swedish Institute supported this work. We thank Birgitta Grape, RN, for her assistance in the follow-up work throughout all these years, Hans Lundström, B.Sc., and Jan Qvist, B.Sc. at Statistics Sweden for help with the age- and gender-matched population derived for comparison with the Biocor population, Dan Lindblom, MD, for advice and assessment of the relative survival data and critical reviewing of the manuscript and Magnus Backheden, M. Sc. for aid with the statistical analysis.




