-
PDF
- Split View
-
Views
-
Cite
Cite
Edvin Prifti, Adrian Crucean, Massimo Bonacchi, Massimo Bernabei, Bruno Murzi, Stefano Vincenzo Luisi, Vittorio Vanini, Early and long term outcome of the arterial switch operation for transposition of the great arteries: predictors and functional evaluation, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 6, December 2002, Pages 864–873, https://doi.org/10.1016/S1010-7940(02)00613-9
Close - Share Icon Share
Abstract
Objectives: The aims of this report were to study the early and late outcome in terms of mortality, freedom from reoperation, predictors for late pulmonary stenosis (PAS) and insufficiency of the neo-aortic valve (AVI) in patients with transposition of the great arteries (TGA) undergoing arterial switch operation (ASO). Materials and methods: Between January 1990 and December 2001, 134 patients with TGA underwent ASO. The patients were divided in Group I (n=88)-TGA with intact ventricular septum and Group II (n=46)-TGA with ventricular septal defect (VSD). The pulmonary artery was reconstructed employing the direct anastomosis technique (PT-I) in 21 (15.7%) patients, the double-patch technique (PT-II) in 41 (30.6%), single pantaloon patch (partial circumference) (PT-III) in 46 (34%) and single pantaloon patch (total circumference) (PT-IV) in 35 (26%) patients. The mean follow-up was 3.4±1.3 years. Results: The hospital mortality was 17 (12.7%) patients. The mortality in Group I was significantly lower than Group II (P=0.002). The overall actuarial survival at 1, 3 and 5 years follow-up resulted to be 98, 93, and 91.5%, resulting to be significantly higher in Group I (P=0.032). The multivariate analysis revealed the complex TGA (P=0.007), VSD (P=0.032), coronary anomalies (P=0.004), aortic coarctation or hypoplastic aortic arch (P=0.021), left ventricular outflow tract obstruction (LVOTO) or moderate PAS (P=0.041) as strong predictors for poor free-reoperation cumulative survival. A strong inverse correlation was found between the mean trans-pulmonary gradient at follow-up and the age at the operation (r=−0.41, P<0.0001). The univariate analysis revealed the PT-I technique (P=0.002), prior moderate PAS (P=0.0001), and age <1 month (P=0.018) as strong predictors for moderate-to-severe PAS. The neo-AVI incidence was significantly higher in Group II (P=0.011). Predictors for neo-AVI were male sex (P=0.003), preoperative neo-AV Z-score >1 (P<0.001), prior or concomitant operation for aortic coarctation or hypoplastic aortic arch (P=0.001), LV retraining (P=0.003). Conclusion: ASO remains the procedure of choice for the treatment of various forms of TGA with acceptable early and later outcome in terms of overall survival and free reoperation. Strong predictors for poor overall free-reoperation survival are complex TGA, VSD, coronary anomalies, aortic coarctation and LVOTO or moderate PAS. The pulmonary artery reconstruction using a single ‘pantaloon patch’ seems to offer less residual stenosis. Patients with a VSD and a significant mismatch between the neo-aortic root and distal aorta are at a higher risk for developing postoperative neo-AVI.
1 Introduction
In the past two decades the arterial switch operation (ASO) has become the preferred surgical procedure for transposition of the great arteries (TGA). After the first successful ASO in 1975 by Jatene [1] and its modification by Lecompte et al. [2], a considerable number of series have been reported [3–13]. In contrast to the atrial switch procedures (Mustard or Senning), the ASO has the advantages of maintenance of sinus node function, preservation of the left ventricle as the systemic ventricle, and the mitral valve as the systemic atrioventricular valve. A limited number of long-term studies revealed an excellent mortality and freedom from re-operation in patients undergoing ASO [3,10–13]. However, in the ASO, the coronary arteries are translocated, the pulmonary valve becomes the systemic outflow valve, and the pulmonary arteries (PA) may be distorted because of the atypical relation between the great vessels. Concerns exist regarding the fate of coronary arteries [14], the function of neo-aortic valve (AV) [10,12,15], and the development of pulmonary artery stenosis (PAS) [10–13].
The aims of this report were to: (1) study the early and late outcome in terms of mortality and freedom from reoperation in patients undergoing ASO; and (2) analyze the predictors for poor early and late survival as well as predictors for late PAS and neo-aortic valve insufficiency (AVI)
2 Materials and methods
Between January 1990 and December 2001, 134 patients with TGA undergoing ASO alone or in combination to other surgical procedure were included in the study. Patients with various forms of TGA undergoing REV, Rastelli operation, Senning procedure, Fontan operation or patients with corrected TGA undergoing combined ASO to Senning procedure, were excluded from this study.
2.1 Patients’ characteristics
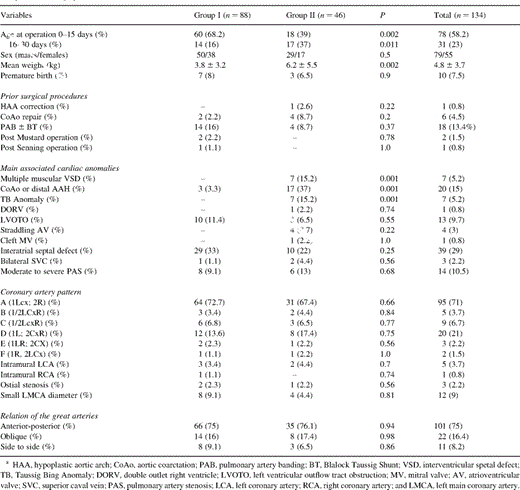
The patients were divided in Group I (n=88)-TGA with intact ventricular septum (IVS) and Group II (n=46)-TGA with ventricular septal defect (VSD). The Group II was divided in: IIA (n=13)-TGA, VSD and one-step coarctation repair, IIB (n=8)-Taussig-Bing anomaly or double outlet right ventricle, and IIC (n=26)-TGA with VSD alone. The patients’ preoperative data are given in Table 1 .
2.2 Preoperative assessment and management
All patients underwent preoperative echocardiography. 47% of them underwent balloon atrioseptestomy. Cardiac catheterization was obligatory in all patients with complex TGA. Prostaglandin infusion before surgery was administered in 56% of cases.
2.3 Operative techniques
Cardiopulmonary bypass (CPB) was instituted using a single venous cannula or bicaval cannulation in patients with VSD. The myocardial protection was achieved by multidose cold crystalloid cardioplegia and in ten patients with blood cardioplegia. Circulatory arrest was used during surgery with additional low flow perfusion if required. The PA were widely mobilized beyond the upper lobe branch. After clamping the ascending aorta, the aorta was divided at the previously marked site, and the main PA was divided just proximal to its bifurcation in cases undergoing coronary transfer after neo-aortic reconstruction and close to the sinutubular junction in cases undergoing coronary transfer before neo-aortic reconstruction. Then, the Lecompte procedure was performed in 91% of cases. The coronary arteries were cut out with a generous aortic button and widely mobilized to avoid tension or distortion, and then reimplanted to the neoaorta. Special techniques such as takedown of the commissure and creation of a button of the aortic wall including the coronary were employed in cases with intramural course of a coronary artery. Different surgical techniques were employed for PA reconstruction such as direct anastomosis (PT-I) without any patch material according to the original description of Pacifico [16], the PA reconstruction using two pericardial patches (PT-II), single pantaloon patch (partial PA circumference) (PT-III) according to Paillole et al. [17] and the single pantaloon patch (total PA circumference) (PT-IV). The VSD was usually closed through the right atrium using the standard techniques, although in different cases with muscular VSD or with complex defects the right ventriculotomy was employed. In two patients with muscular VSD, the defect was closed through a right atriotomy. The closure of a perimembranous defect associated with the malalignment of septal components was performed through the PA in three patients. In patients with aortic coarctation or with hypoplastic aortic arch, the repair of the aortic arch was performed concomitantly to ASO. The operative data are given in Table 2 .

2.4 Postoperative care
Inotropic support included dopamine (5 μg/kg per min) and low dose of epinephrine for the first 12 postoperative h. All the postoperative complications were carefully recorded (Table 2).
2.5 Follow-up
Survival status was determined in all patients by contacting parents by telephone. Follow-up ranged from 8 to 144 months, mean 3.4±1.3 years. Clinical follow-up data were obtained by means of direct contact with the referring paediatric cardiologist. A complete clinical follow-up, including echocardiographic examination, was performed in 95 (88%) patients from 108 survivors. Thirteen contacted patients who did not undergo echocardiographic examination were excluded from the study.
2.6 Definitions
Coronary anomalies were considered coronary arteries with conus branch, ostial stenosis and intramural coronaries. Intramural coronary artery was defined a coronary vessel crossing a commissure and the coronary orifice did not have a cone. The gradient progression was measured as a difference between the mean transpulmonary gradient (M-TPG) at follow-up and at discharge for each patient. Patients presenting associated perimembranous VSD, muscular VSD, Taussig-Bing anomaly, double outlet right ventricle, and those undergoing concomitant aortic coarctation or hypoplastic aortic arch repair were categorized as complex TGA.
2.7 Statistical analysis
Group statistics were expressed as mean±1 standard deviation. The generalized Wilcoxon test was performed for the statistical analysis between groups. Fisher's exact test was used for the non-continuous variables. The relationship between preoperative and postoperative variables within the same group was assessed by the McNemar test. The multivariate logistic regression and the Cox regression (BMDP2L software) models were employed appropriately. Long-term survival rates were calculated using the Kaplan–Meier method and statistical significance was calculated by the log rank test. The Spearman linear regression test was employed for analysing the correlation between variables. Significance between data was considered achieved when P<0.05.
3 Results
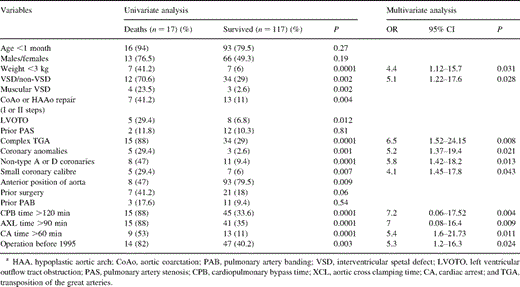
The hospital mortality was 17 (12.7%) patients. The mortality in Group I was significantly lower than Group II (P=0.002). Within the Group II, the mortality resulted to be significantly higher in Group IIA [four (44%) patients] versus Group I (P=0.004). The CPB, aortic cross-clamping and cardiac arrest times were significantly higher in Group II. The left ventricular (LV) dysfunction incidence resulted to be significantly lower in Group II (P=0.034). The postoperative myocardial infarction was found in six (13%) patients in Group II versus two (2.3%) in Group I (P=0.034) (Table 2).
The multivariate logistic regression analysis revealed the weight <3 kg (P=0.031), associated VSD (P=0.028), complex TGA (P=0.008), non-type A or D coronary arteries (P=0.013), coronary anomalies (P=0.021), small diameter coronary artery (P=0.043), CPB time (P=0.004), aortic cross-clamping time (P=0.009), cardiac arrest >60 min (P=0.011) and operation year <1995 (0.024) as strong predictors for poor operative survival (Table 3) . Two patients necessitated coronary revascularization due to significant stenosis of both coronary arteries [18].
The overall actuarial survival at 1, 3 and 5 years follow-up resulted to be 98, 93, and 91.5% and the free reoperation actuarial survival was 95, 90.5 and 83% (Fig. 1A) . From 117 followed patients, 34 were in Group II. The actuarial survival was significantly higher In Group I (P=0.032) (Fig. 1B). Three patients died due to pulmonary vascular disease (Group II), three other patients died of progressive heart failure after reoperation (Groups I and II), one patient died after coronary revasculazarion due to myocardial infarction, one patient due to non-cardiac causes and another one unknown. The type and number of reoperations during follow-up are given in Table 4 . The reoperation incidence resulted to be higher in Group II (nine patients) versus Group I (six patients) (P=0.012). The multivariate analysis revealed the complex TGA (P=0.007), VSD (P=0.032), coronary anomalies or non-type A or D (P=0.004), aortic coarctation or hypoplastic aortic arch (P=0.021), LV outflow tract obstruction or moderate PAS (P=0.041) as strong predictors for poor free-reoperation cumulative survival (Table 5) .
Actuarial survival analysis. (A) Actuarial survival and freedom from reoperation survival. (B) Actuarial survival according to the presence of preoperative interventricular septal defect. Legend: AS, actuarial survival; AS-R, free reoperation actuarial survival; TGA-IVS, transposition of the great arteries with intact ventricular septum; and TGA-VSD, transposition of the great arteries with ventricular septal defect.
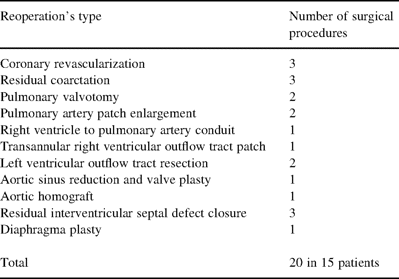
Total number of all surgical procedure performed during follow-up
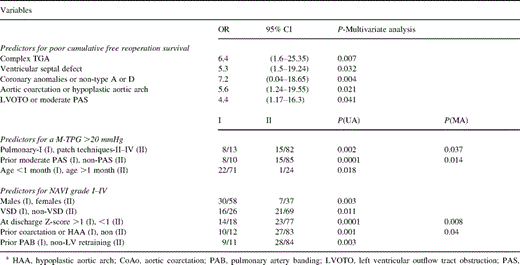
At mean 2.3±0.6 years after ASO the mean trans-pulmonary gradient was 12.8±5.4 (mmHg) versus 5.6±4.3 (mmHg) at discharge (P<0.001). Four patients developed severe PAS. Twenty-three patients had a mean trans-pulmonary gradient >20 mmHg at follow-up. The followed patients were divided according the employed technique for PA reconstruction (Fig. 2) . At discharge there were no differences regarding the residual mean trans-pulmonary gradient between groups in relation to the employed technique. At follow-up, the mean trans-pulmonary gradient increased significantly in all groups versus the discharge values and was significantly higher in patients undergoing PT-I technique versus PT-III (P=0.011) and PT-IV (P=0.003). Mean trans-pulmonary gradient progression was 11.3±4.2 (mmHg) (PT-I), 9.2±3.3 (mmHg) (PT-II), 6.5±3.6 (mmHg) (PT-III) and 4.8±3.4 (mmHg) (PT-IV), resulting to be significantly higher in patients undergoing PT-I or PT-II versus patients undergoing PT-III or PT-IV (Fig. 2). A strong inverse correlation was found between the mean trans-pulmonary gradient at follow-up and (1) the age at the operation (r=−0.41, P<0.0001) (Fig. 3) ; and (2) PA Z-score (r=−0.87, P<0.0001). The univariate analysis revealed the PT-I technique (P=0.002), prior moderate PAS (P=0.0001), and age <1 month (P=0.018) as strong predictors for moderate to severe PAS at follow-up (Table 5).
Mean gradient through the pulmonary artery anastomosis at follow-up according to the employed surgical technique for pulmonary artery reconstruction. Legend: Pulmonary technique-I (PT-I) – 13 followed patients. Pulmonary technique-II (PT-II) – 29 followed patients. Pulmonary technique-III (PT-III) – 37 followed patients. Pulmonary technique-IV (PT-IV) – 16 followed patients.
Correlation between the age at operation and mean transpulmonary gradient at follow-up.
At mean 2.3±0.6 years after ASO, the neo-AVI was present in: one patient-grade IV, two patients-grade III, seven patients-grade II and 27 patients-grade I. From 95 patients with completed follow-up, 26 patients were in Group II and 69 patients in Group I. The incidence of the neo-AVI was significantly higher in Group II (P=0.011). Other predictors resulted to be the male sex (P=0.003), preoperative neo-AV Z-score >1 (P=0.0001), prior or concomitant operation for aortic coarctation or hypoplastic aortic arch (P=0.001), LV retraining (P=0.003) (Table 5).
4 Discussion
The ASO is the procedure of choice in the treatment of various forms of TGA, although, the assessment of late mortality and morbidity predictors with special regards to the neo-AV function, the reconstructed PA as well as the fate of the reimplanted coronary arteries are required prior to any judgement. Few reports analyzed these parameters within the same series [10–13].
This study confirms that ASO can be accomplished with acceptable early and late outcome in patients with simple TGA. The early postoperative mortality and morbidity in this cohort was higher than the recently reported series [10–12], probably due to a higher incidence of complex TGA and associated anomalies. Such outcome improved significantly during the last six years, probably related to a ‘learning curve’ with treatment of complex TGA and rare coronary artery patterns, employment of new techniques [7,19], improvement of perioperative care and CPB management. The predictors’ analysis for poor operative survival was similar to the reported data [10,11,13]. The intraoperative predictors such as CPB, aortic-cross clamping and cardiac arrest times were related to the presence of complex TGA or necessity of associated surgical procedures. In difference to other studies, we did not find the LVp/RVp ratio a strong predictor for poor early postoperative outcome. This, probably due to an improvement of patient selection process and LV retraining, in patients with a lower LVp/RVp ratio. The main reason of death in our series was in general myocardial infarction due to imperfect coronary arteries translocation. We found the coronary anomalies and coronary artery distribution with a single left or right ostium as strong predictors for poor postoperative outcome confirming the others’ experience [20]. The coronary related mortality was significantly higher in our initial experience. Later on, with surgical techniques improvement (a more extensive epicardial mobilization of the proximal arteries, modifications of the implanted site, reimplantation of the coronary arteries after aortic reconstruction), such a mortality was reduced significantly. Despite the surgical techniques improvement, the incidence of myocardial ischemia or infarction remains still high [10]. Interestingly, in our experience, we found late coronary arteries obstruction necessitating revascularization procedures in patients with excellent early postoperative outcome [18], this probably due to the development fibrous tissue at the coronary button suture lines, or due to a moderate vessel ‘kinking’ secondary to an imperfect translocation. We believe, that the coronary artery pattern in TGA, remains a delicate issue regarding the treatment of this malformation. The univariate analysis demonstrated that the one-step aortic coarctation or hypoplastic aortic arch repair was a risk factor for increased postoperative mortality. The opinion regarding this approach is still controversial. Wernovsky et al. [13] found an increased mortality in patients undergoing concomitant aortic repair to ASO, similarly to other studies [10,21]. Other groups reported excellent outcome in small cohorts undergoing one stage repair [18]. Our experience with this approach is quite limited. We believe that a one-step repair may have excellent results after a considerable ‘learning curve’.
The overall survival and free reoperation survival at 5-year follow-up were 91.5 and 83%, respectively, similar to the reported series [10–12]. Strong predictors for poor cumulative free-reoperation survival were the presence of the VSD, coronary anomalies, preoperative LV outflow tract obstruction or moderate PAS, aortic coarctation and complex TGA forms. The most common cause of late death was related to the pulmonary vascular disease, found in patients with VSD, which contributes to pulmonary volume overload before surgery [10]. Almost 15 survivors necessitated reoperation during follow-up and ten of them survived. The causes of late reoperation were mainly related to a significant trans-pulmonary gradient, neo-AVI and residual VSD or aortic coarctation, similarly to the previously reported series [10–12].
The PAS is widely recognized as the most frequent complication after ASO [10–13,21–22]. Different mechanisms have been implicated. In our series we found a strong inverse correlation between age at operation and late development of moderate-to-severe mean trans-pulmonary gradient, confirming the hypothesis that this gradient commensurates with significant somatic growth. It seems that in neonates there is inadequate growth of the PA resulting in flattening of the reconstructed right ventricular outflow tract. But mainly, this inadequate somatic growth is localized at the suture line, probably secondary to the development of fibrous tissue, causing a circumferential narrowing. Other possible mechanisms may be the presence of ductal tissue which may cause the so called ‘coarctation’ of the left PA and the ‘bowstring’ of the left PA over the aorta after the Lecompte procedure causing significant flow asymmetry. Another cause might be the employed technique for PA reconstruction. The tension at the anastomotic site has been implicated as a possible adjunctive factor to inadequate somatic growth at the suture line. Such a tension is higher when a direct anastomosis between distal and proximal PA is performed or when the mobilization of the distal PA is not extensively done. The comparison of the mean trans-pulmonary gradient revealed a significant gradient progression, and as a consequence greater stenosis, at follow-up in patients undergoing direct anastomosis technique. Also such a progression was higher even in patients undergoing double-patch technique compared to patients undergoing the ‘pantaloon patch’ techniques, this probably due to the fact, that with the double patch technique are filled only the defects left by coronary explantation. Indeed, the single ‘pantaloon patch’ technique provides additional length and tissue quantity to the proximal neopulmonary root. The stenosis seems to progress slower in patients undergoing complete ‘pantaloon patch’ technique, which provides enough tissue length for a complete pulmonary circumferential anastomosis.
Despite, the anatomic PA valve becomes the neo-aortic systemic valve after ASO, the fate of such anatomic component is not well studied. Different studies demonstrated a progressive and significant increase of the insufficiency grade of the neo-AV postoperatively [6,21]. Yamaguchi et al. [6] have reported that the two-stage ASO was associated with a higher incidence of neo-AVI. We found a similar result in patients undergoing PA banding before ASO. The authors’ opinion regarding the mechanisms of late neo-AVI is controversial. During ASO, the infringement of the neo-aorta occurs only at the sinus and sinutubular junction. Hutter et al. [15] reported an ongoing increase of the neo-AV and sinus-tubular junction diameters, regressing to a more normal values at 6–10 years postoperatively, however the sinutubular Z-score increased more than the neo-AV Z-score. Other studies reported a progressive aortic root dilatation over time when commensurate with the somatic growth [23]. In our series, a significantly higher neo-AVI incidence was found in VSD patients. Such findings indicate that the post-ASO neo-AVI is probably related to an increased preoperative flow across anatomic pulmonary valve in such patients, resulting in a more pronounced mismatch between the neo-aortic root and distal aorta. Blume et al. [24] postulated another hypothesis regarding to a real aneurysmatic dilatation at the neo-aorta anastomotic site extended towards the aortic sinus probably caused by turbulence at the mismatched anastomosis. Hazekamp et al. [25] found in another patient undergoing switch back procedure at 40 months after ASO, the aortic root aneurysm as the main cause of neo-AVI. In our experience, both patients undergoing reoperation for neo-AVI, presented intraoperatively an enlarged neo-aortic root and bulging sinus. We hypothesize, that the mechanism of the post-ASO neo-AVI consists mainly in two phases: phase 1-the increased preoperative flow through the neo-AV, causes an increase of the entire neo-aortic root diameter prior operation, but mainly of the neo-AV diameter; phase 2-the mismatched neo-aortic anastomosis induces flow turbulence and as a consequence, mainly sinus dilatation preventing leaflet coaptation. However larger series of patients and longer follow-up are required for a proper evaluation of the postoperative neo-AV functional status and residual PAS.
4.1 Conclusion
ASO remains the procedure of choice for the treatment of various forms of TGA with acceptable early and later outcome in terms of overall survival and free reoperation. Strong predictors for poor overall free-reoperation survival are complex TGA, VSD, coronary anomalies, aortic coarctation and LV outflow tract obstruction or moderate PAS. The reconstruction of the pulmonary root employing a single ‘pantaloon patch’ seems to offer less residual pulmonary artery stenosis. Patients with a VSD and a significant mismatch between the neo-aortic root and distal aorta are at a higher risk for developing postoperative neo-AVI.
Dr F. Lacour-Gayet (Le Plessis-Robinson, France): I would like to ask you a question about the pulmonary stenosis. Do you identify other factors than the material, namely the length of the aorta, as being a potential cause of pulmonary stenosis?
Dr Prifti: According to our point of view and the analysis that we performed, there are two main mechanisms for the postoperative pulmonary stenosis. The main mechanism is the tension at the anastomotic site. Certainly, when the main pulmonary artery is shorter than the aorta, there is an adjunctive tension at the anastomotic site, even when a wide mobilization is performed. However, we did not take into consideration the length of the aorta in our study. On the other hand, this study demonstrates that the direct anastomosis induces further tension on the anastomotic site. The second thing, is that the employed Prolene suture in usually 1-week-old babies, induces an inadequate growth at the anastomotic pulmonary site, which in our point of view is another mechanism for late pulmonary artery stenosis.
References
- aorta
- aortic arch
- aortic coarctation
- pulmonary artery stenosis
- left ventricular outflow obstruction
- transposition of great vessels
- pulmonary artery
- arterial switch operation
- lung
- ventricular septal defect
- anastomosis, surgical
- constriction, pathologic
- follow-up
- hospital mortality
- preoperative care
- reconstructive surgical procedures
- repeat surgery
- mortality
- interventricular septum
- patch
- distal zone of aorta
- cumulative survival rate
- univariate analysis
- mismatch




