-
PDF
- Split View
-
Views
-
Cite
Cite
G. Stellin, V.L. Vida, O. Milanesi, M. Rubino, M.A. Padalino, S. Secchieri, G. Pittarello, D. Casarotto, Surgical treatment of complex cardiac anomalies: the ‘one and one half ventricle repair’, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 6, December 2002, Pages 1043–1049, https://doi.org/10.1016/S1010-7940(02)00669-3
Close - Share Icon Share
Abstract
Objective: One and one half ventricle repair is a surgical option for congenital cardiac anomalies characterised by right ventricle (RV) hypoplasia and/or dysplasia. Methods: From March 1994 to March 2001, eight patients (mean age 9.1 years, range 7 months to 35 years) with hypoplastic and/or dysplastic RV underwent correction of their intracardiac anomaly in association with a BCPS (one and one half ventricle repair). Preoperative diagnoses included: Ebstein's anomaly of tricuspid valve (TV) in two, inlet ventricular septal defect (VSD) in association with straddling/overriding TV in two patients, pulmonary atresia-intact ventricular septum in one, tertralogy of Fallot in association with complete atrioventricular canal defect in one, truncus arteriosus in one and heterotaxy syndrome with VSD and anomalous systemic venous return in one. Four patients underwent previous surgery which included: main pulmonary artery (MPA) banding in two patients, pulmonary valvotomy, central shunt and right ventricular outflow tract reconstruction in one, pulmonary artery separation from truncus arteriosus and modified Blalock–Taussig shunt in one, and MPA closure in one. Two patients underwent a bidirectional cavo-pulmonary shunt before the one and a one half ventricle repair. Associated cardiac lesions were treated simultaneously. Results: There were no hospital deaths. All the patients were discharged home in good clinical conditions. There were no late deaths or reoperations. At mean follow-up of 29.8 months (range 8 months to 7.3 years) all the patients are alive and in good general conditions. MPA percutaneous balloon dilation was performed in two patients at 33 and 4 months, respectively, both after MPA reconstruction (which was previously ligated) and dilation of the left pulmonary artery branch in one patient, repeated twice at 10 and 14 months from repair, for a hypoplastic left pulmonary artery after truncus arteriosus repair. Conclusions: Surgical treatment of congenital cardiac anomalies in the presence of a hypoplastic and or a dysplastic RV by means of one and one half ventricle repair has the advantage of reducing the surgical risk for biventricular repair, and compared to the Fontan circulation, it maintains a low right atrium pressure, a pulsatile pulmonary blood flow and improves the systemic oxygen saturation. Short and medium-term results are promising. Longer follow-up is needed, to prove the efficacy of such a repair, in the long term.
1 Introduction
Right ventricular hypoplasia and/or dysplasia is found in association with a wide spectrum of congenital anomalies. In these cardiac defects, the left ventricle is usually normal while the right ventricle (RV) may not be capable of completely supporting the pulmonary circulation [1]. However, the RV pumping power may still be utilised to sustain the pulmonary circulation, provided its physiological tolerance is not acceded [2].
Since the first original work published by Billingsly et al. in 1989 [3], the purpose of the so-called ‘one and one half ventricle repair’ is to achieve a physiological correction by separating the pulmonary circulation from the systemic one, maintaining a pulsatile blood flow in the pulmonary arteries. This is obtained by ‘unloading’ the insufficient RV at the time of the correction, in diverting the superior vena cava (SVC) blood to return directly into the pulmonary arteries by means of a bidirectional cavopulmonary shunt (BCPS) (Azzolina's procedure) [4,5]. In this way, the RV work is reduced to about 30% [1] and the impaired ventricle becomes adequate to its function.
In order to analyse the short and middle-term results of such a repair, we have reviewed our surgical experience with congenital anomalies where we have combined an Azzolina procedure to an intracardiac repair.
2 Material and methods
Included in this study are eight patients with multiple varieties of congenital heart defects including also a hypoplastic and/or dysplastic RV. There were six males and two females with a median age of 3 years (range 7 months to 35 years) who underwent one and a half ventricle repair between March 1994 and March 2001, at our Institution (Table 1 ).
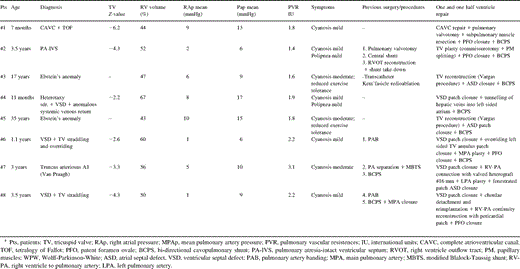
All the patients were studied preoperatively by means of a two-dimensional (2D)-echocardiography and Doppler. Cardiac catheterisation was also performed in all, before repair, with the main purpose of measuring pulmonary artery pressure and calculating pulmonary vascular resistances (PVR, Table 1). Previous palliation to optimising pulmonary blood flow was performed in four patients, consisting of a central shunt combined to a pulmonary valvotomy in patient #2 (Table 1); a modified right Blalock–Taussig shunt in adjunct to pulmonary artery separation to common trunk in patient #7 (Table 1); pulmonary artery banding in two patients #6 and #8 (Table 1).
A BCPS was performed as a palliative procedure in patients #7 and #8. One-stage repair was achieved in four of them (patients #1, #3, #4, and #5, Table 1). In patient #3 with an Ebstein tricuspid valve (TV) anomaly and recurrent supraventricular tachycardia (patient #3, Table 1) a transcutaneous Kent fascicle radioablation was performed successfully 6 years prior to the repair.
2.1 RV assessment
In each patient the TV and the RV volume were carefully studied prior to repair with the aim of planning a proper surgical strategy. TV assessment was made by obtaining the larger annulus diameter measured from a standard 2D-echocardiography apical four chamber view. This was indexed for body surface area and then compared to normal values according to Rowlatt et al. [6]. The TV Z-value score [7] was then calculated (except for patients #3 and #5 of Table 1 with an Ebstein TV). and was found to be between −2.5 and −6.25, with a median of −3.3.
The RV volume was also assessed in all, before repair, with a modified Tomita method [8] and the value varied from 44 to 67% of normal with a median value of 51%.
Patients #3 and #5, with an Ebstein anomaly, presented clinically with moderate cyanosis and reduced exercise tolerance. A mild to moderate cyanosis was the main presenting symptoms in all the remaining six patients, with an associated mild polipnea in patients #2 and #4, Table 1.
2.2 One and one half ventricle repair
Surgical repair was carried out through a midline sternotomy. Previous systemic-to-pulmonary shunts were controlled in patients #2 and #7 and divided during cardiopulmonary by-pass. SVC and inferior vena cava (IVC) were cannulated directly (SVC next to the origin of left innominate vein). Moderate hypothermic (rectal temperature between 22 and 26°C) cardio pulmonary by-pass was instituted in all. Aortic cross-clamping and multiple doses of haematic (crystalloid in patient #1) cardioplegia was employed in all. In all the patients, the RV cavity was inspected and found to be hypoplastic. The TV was sized with hegar dilators confirming the preoperative echocardiographic measurements. TV reconstruction, according to Vargas et al. [9], was performed in two patients with an associated Ebstein anomaly (patients #3 and #5, Table 1). In patient #2 treated previously with right ventricular outflow tract (RVOT) reconstruction after central shunt and pulmonary valvotomy for pulmonary atresia and intact septum (PA-IVS), at the time of repair we performed in addition a TV commissurotomy and splitting of the papillary muscles. In patient #6 with an overriding TV across an inlet type ventricular septal defect (VSD) together with a VSD patch closure, the left ventricular (LV) component of the straddling TV was obliterated with patch in order to divert the IVC venous return only into the RV. In patient #8 with an almost absent ventricular septum (large inlet and large conoventricular VSD) and a second degree straddling TV, the tensor apparatus implanting in the LV cavity was carefully detached and reimplanted onto the VSD patch. Direct main pulmonary artery (MPA) reconstruction was necessary in patient number #6, Table 1 to restore the connection between MPA, previously ligated, and branches. The azygos vein was ligated in two of them, at the time of the BCPS as a staging procedure and in six of them at the time of repair, to avoid blood steal from the SVC to the IVC, due to the pressure gradient between the two systems. An atrial septal communication, when present, was totally closed in all, apart for patient #7 were a large ASD that was reduced to 4 mm size by a fenestrated patch.
In all of them the RA and the SVC mean pressures were continuously monitored in the immediate postoperative course.
Follow-up was completed and updated in all, with a mean of 29.8 months (range, 8 months to 7.3 years). All the patients were followed with clinical evaluations, 2D-ecocardiography with Doppler study of the SVC and IVC blood flow. Cardiac catheterisation was performed in three patients with direct measurement of the pressure of the RA and SVC. Exercise stress test at cicloergometer was performed in the two adult patients in our series, both with Ebstein's anomaly of the TV.
3 Results
We managed to achieve complete separation of the systemic circulation from pulmonary circulations in all, apart for #7 with increased PVR.
There were neither early deaths nor reoperations. The immediate postoperative mean SVC pressure at the time of the discharge from intensive care unit ranged between 12.1 and 16.2 mmHg (mean of 13.8 mmHg). There was a phasic SVC blood flow reversing in systole with a mean peak pressure of 20 mmHg (ranging from 18 to 24 mmHg). Mean RA pressure was 6.3 mmHg, ranging from 6 to 9 mmHg. Postoperative complications included: chylopericardium treated with drainage and intravenous hyperalimentation for 22 days in one (patient #4); RV wall thrombosis after Vargas repair for Ebstein anomaly treated with oral anticoagulation, which spontaneously disappeared at 2D-echocardiographic control performed 20 days later in patient #5 and right pleural effusion requiring drainage in patient #8. Transcutaneous arterial saturation was above 97% in all apart from patient #7 with a fenestrate patch ASD closure (arterial oxygen saturation 88%).
All the patients were discharged home in good haemodynamic conditions and sinus rhythm.
3.1 Follow-up
We have followed all of them with periodical clinical and echocardiographic controls, 8 months to 7.3 years (mean 29.8 months) after discharge. There were no late deaths and no reoperations. Seven patients were in NYHA class I and one in NYHA class II (patient #7). Echocardiographic examination showed good LV function, absent or mild tricuspid and pulmonary valve regurgitation and a pulsatile antegrade flow across the pulmonary valve in all. SVC Doppler evaluation, showed in all a systolic reverse flow simultaneous to the RV ejection and an antegrade flow during the rest of the cardiac cycle. IVC Doppler interrogation disclosed a normal pattern with low amplitude reverse flow during the atrial systole and an antegrade flow during the rest of the cardiac cycle. Interventional cardiac catheterisation was performed in three patients, 4 months, 7.4 months and 5 years (mean 23.8 months) after correction for residual lesions. Percutaneous balloon dilation of the MPA was performed in two patients (#1 and #8) 33 and 4 months, respectively, both after MPA reconstruction (which was previously ligated) and dilation of the left pulmonary artery branch in one patient (#7), repeated twice (at 10 months and at 14 months from repair), for an hypoplastic left pulmonary artery after truncus arteriosus repair. Mean RA pressure was found to be between 6 and 7 mmHg. This was compared to the SVC pressure which was 12, 14 and 24 mmHg, respectively (this last value belongs to patient #7 with elevated PVR). Exercise stress test was performed in two adult patients at (patients #3 and #5) 39 and 19 months, respectively, after repair (who had moderately reduced exercise tolerance preoperatively) and was within normal limits (test interrupted at 125 W for exhaustion) in both of them. No arrhythmias were detected during the entire examination.
4 Discussion
In repairing congenital cardiac malformations, there are situations in which the RV may be inadequate to convey the entire cardiac output to the pulmonary circulation because of its cavity size and/or function. In unloading the RV during the repair by means of an Azzolina operation, the poor outcome for biventricular repair is avoided [1,2]. In addition, in maintaining the insufficient RV as a pumping chamber for the pulmonary circulation at the time of separation of the two circulations, the well-known threatening complications of the Fontan circulation seems to be avoided.
In our limited experience, one and one half ventricle repair can be achieved with a very low surgical risk, even in the presence of associated complex congenital cardiac anomalies. We are convinced that the surgical strategy for one and one half repair needs to be planned in detail before the repair, as we did with our experience. In fact, in none of our patients a cavopulmonary connection was created as a ‘salvage procedure’ in treating an acute RV dysfunction during the attempt of a biventricular repair.
In two of our patients (patients #7 and #8, Table 1), the Azzolina procedure was performed in a stage prior to the intracardiac repair. In both of them, at the time of the cavopulmonary connection, we were still considering a stadiation towards a Fontan-type of repair: However, at the pre-repair catheterisation, in patient #7 we found that PVR were too high (3.1 U/m2) to consider a total cavopulmonary connection. One and one half ventricle repair was performed as an alternative to heart transplantation. In patient #8, who underwent MPA banding for an almost complete absence of the interventricular septum and II degree TV straddling, a detailed echocadiographic transthoracic and transesophageal TV assessment in older age, convinced us to attempt a ventricular septation, regardless of the TV tensor apparatus crossing an inlet-type VSD. No residual septal defects and only a mild TV incompetence is present, at the follow-up control.
Chronic cyanosis and reduced exercise tolerance were the main complaints in our patients with an Ebstein anomaly. In both of them a large right-to-left shunting at atrial level was ‘naturally unloading’ an hypoplastic and dysplastic RV but producing an important cyanosis. At correction, in reducing the systemic venous return to the RA of one-third, we were entitled to perform a more aggressive TV reconstruction which downsized the TV annulus to a Z-value less then 0, however, obtaining an optimal TV competence. Total atrial septation was thereafter completed leading to a postoperative normal arterial oxygen saturation, in both of them. Exercise stress test performed at the follow-up control showed normal tolerance to effort in both.
In patient #2, who underwent repair of PA-IVS by means of a RVOT patch reconstruction after a pulmonary valvotomy and central systemic-to-pulmonary artery shunt, in adjunct to a BCPS we performed a valve TV commissurotomy and papillary muscles splitting. In fact, to an hypoplastic TV annulus (Z-value, −4.3) we have found that a thick and sclerotic subvalvar apparatus was further limiting the blood inlet to the RV. Papillary muscles splitting as far down as the RV wall allowed a better movement of the tensor apparatus, improving the leaflets excursions and therefore the valve performance. There is only a mild TV incompetence at the follow-up echocardiographic control.
Our follow-up and that one of other reported experience [1,2,10–15] is still too short to advance any speculation as far as the long-term results with this repair is concerned. However, we are all aware of the potential complications after a long standing total cavopulmonary connection [16]. Indeed, the ‘Achille's heel’ of the Fontan circulation is the IVC hypertension. Chronic liver congestion with chest effusions and ascites, protein loosing enteropathy, atrial arrhythmias, atrioventricular valve regurgitation, systemic ventricular failure are all well-known life-threatening complications which motivated us to attempt one and one half ventricle repair, whenever possible.
We believe that the main goal of this repair is to achieve a complete separation of the pulmonary from the systemic circulation maintaining a low pressure in the IVC district. In our patients the mean postoperative RA mean pressure at the time of discharge from intensive care unit was 7 mmHg, including one patient (patient #7, Table 1) with increased PVR. This compares favourably with the mean SVC pressure recorded at the same time (14 mmHg) and the IVC pressure reported after Fontan circulation [16]. Indeed, the kinetic force produced by a hypoplastic and or dysplastic RV, which is not capable to support the whole cardiac output, can still be utilised with the main purpose of producing a low pressure in the IVC district.
So far, it is not clear whether a laminar pulmonary blood flow (as it is in the Fontan circulation) will hamper the pulmonary microcirculation creating dysfunction, in the long term. However, it is obvious that a pulsatile pulmonary blood flow is more physiological than a continuous one. Systemic SVC hypertension is well tolerated and, so far, there are no complications in our experience and in others reported in the literature [1–3,10–15].
Longer follow-up is needed to evaluate the effect of a combined pulsatile pulmonary arterial blood flow with a systemic venous one. In our population, we have monitored postoperatively the SVC blood flow and, as others [1,2], we have found, that there was a phasic flow with a reverse in systole during the hospital stay which progressed to only a cessation of flow at the time of the follow-up control in all.
In conclusion surgical treatment of congenital cardiac anomalies in the presence of a hypoplastic and or a dysplastic RV by means of one and one half ventricle repair has the advantage of reducing the surgical risk for biventricular repair, and compared to the Fontan circulation, it maintains a low RA pressure, a pulsatile pulmonary blood flow and improves the systemic oxygen saturation. Short and medium-term results are promising. Longer follow-up is needed to prove the efficacy of such a repair, in the long term.
Dr A. Corno (Lausanne, Switzerland): I have to thank you because you underlined this very good surgical option for borderline ventricle not only in terms of size and morphology but also in terms of function. In order to answer to your last question, maybe you know that in Lausanne we just opened an International Registry for 1 and 1/2 ventricular repoair to collect as much as possible data on this type of procedure to see the long-term results. I have two quick questions.
In the group with Ebstein's malformation, what we do is to try to reduce as much as possible tricuspid valve regurgitation and we are very aggressive in the plasty of the tricuspid valve, particularly because we know then only two-thirds of systemic venous return will be allowed through this valve. I would like to know if you do the same.
Dr Vida: I agree with you, we connect also the inferior vena cava with the ventricle so we can be very aggressive in treating the insufficiency of the tricuspid valve. And while the fact that these three patients were only with moderate to severe cyanosis, the only way to turn them pink is to close ASD. So we try to have no regurgitation of the tricuspid valve, so we are very aggressive in treating it.
Dr Corno: And the other question, in the group with massive tricuspid valve regurgitation, we recently had a patient in whom we did a tricuspid valve repair, but because of the very poor right ventricular function, we associated the cavopulmonary connection. So it was a one and a half ventricle. And after a few weeks we had a complete recovery of the right ventricular function with pulsatile flow in the right pulmonary artery and systolic retrograde flow into the superior vena cava. We had to go back and narrow the origin of the right pulmonary artery. I wonder if you had the same experience.
Dr Vida: Well, I have one more slide to show, if I can. We evaluated the 2D echocardiography of the superior vena cava, and we showed that we have a peak of systolic reverse flow.
(Slide) We have a reverse flow soon after the QRS wave here, and from the other rest of the cardiac cycle we have an antegrade flow throughout the superior vena cava. And so I saw that the peak in the superior vena cava goes from 20 to 24, the peak, and the median was 18.
Well, we can see that I said in the conclusion that we have a moderate elevated hypertension in the superior vena cava and we have no complication until now.
Dr T. Spray (Philadelphia, Pennsylvania, USA): This was a very nice series. Obviously, it is difficult to define the optimal election of patients for the use of this technique. I also wonder what is the longterm need for this type of approach. I wonder now that you have had the chance to look at these patients over the course of several years, if you would ever consider taking down this connection and going back to a two-ventricle repair. If the right atrial pressure is only 6 or 7 mm at follow-up examination, maybe some of these patients can be “staged” to a two-ventricle repair. Have you looked at the longterm follow-up in terms of caval pressures in the inferior vena caval portion of the connection to see if takedown might be possible?
Dr Vida: Well, we reviewed three patients with cardiac cath, and we measured also the pressure in the superior vena cava, and then it goes with a mean of 10 to 12 mmHg. However, we evaluated only in three patients the Z value of the tricuspid valve, and the Z value does not differ significantly from the value at the time of operation. So we have a little increase in the ventricular volume, but it is very difficult to tell right now if you can go back to a biventricular repair.
Dr G. Sarris (Athens, Greece): This presentation explores a borderline situation in anatomy and physiology that we may encounter. The results are very nice. I have two questions. The first is, have you identified a group of patients in whom you know before surgery that this will be a planned one-and-a half ventricle approach, or do you plan and hope for a two-ventricle repair and then, in some cases back out if the post-repair hemodynamics are unsatisfactory in the operating room and perfom a bidirectional Glenn?
Dr Vida: May I answer the first question?
Dr Sarris: Certainly.
Dr Vida: In all of our series, in all the patients we proposed the one and a half ventricle repair. In fact, as I told you, we measured the Z value, the volume, then we measured the pulmonary resistance. We have only two patients that have previously the bidirectional Glenn to be repaired. In that case we know whether or not to go until a Fontan operation or a one and a half ventricle repair. And then one case we closed the main pulmonary artery and then we reconstructed it. And the other case, we had a separation of the pulmonary artery from the common trunk, and then we had reimplantation. All the patients are proposed for a one and a half ventricle repair. There was no failure in the OR and then we turn back to the one ventricle.
Dr Sarris: My second question, as a follow-up along this line of reasoning pertains to patients with Ebstein's malformation. Is it fair to say that you have decided to employ the one-and-a-half ventricle approach as surgical therapy for the type 4 severe Ebstein malformation?
Dr Vida: In all the three cases the ventricular volume is under 50% of normal value, so it is unthinkable in a biventricular repair, and in order to prevent the one-ventricle repair versus a Fontan procedure, we proceeded, versus a one and a half ventricle repair.
In our experience, and in answer also to Dr. Spray, one of the most important things in this correction is the low right atrial pressure after surgery. This is versus the Fontan repair where we have an overcharged hepatic and IVC district. Here we have all the atrial pressure that goes between 6 and 7 mmHg, and this I think is the most important factor in this type of correction and to go back to a biventricular correction where we can go back also in the hepatic parameter I don't think is possible.
Dr S. Sano (Okayama, Japan): I might miss the tricuspid valve size in your presentation. Did you mention it?
Dr Vida: Sure, sure.
Dr Sano: We presented a paper about the surgical treatment of pulmonary atresia with intact ventricular septum in last STS meeting. I think the decision of the procedure is dependent on the size of the tricuspid valve rather than the siyeof the right ventricle. Do you have any data about the relationship between the size of the tricuspid valve and the surgical procedure, for example in which size of the tricuspid valve do you perform biventricular repair, one and one half ventricular repair and one ventricular repair to these patients?
Dr Vida: Well, I think this is a good question. We have 10 patients. The range interval goes between −2.5 to −6.25; however, in nine patients the range goes between −2 and −4.3. In one patient early in our experience we treated with common AV canal Fallot, he is −6.25, and he is probably in the lower limit of this kind of correction I think.
Dr T. Tlaskal (Prague, Czech Republic): I would like to ask you, haven't you observed the development of collaterals between the SVC and IVC? And then, at what age would you do this one and a half repair? Would you do it in an infant?
Dr Vida: We didn't see any collaterals between the SVC and IVC, no.
Dr Tlaskel: And as far as the age is concerned, would you do a one and a half repair in an infant?
Dr Vida: No, no. In this series we considered also two patients, one of 18 years old and one of 35 years old, and they are two patients with absent tricuspid valve.
This article represents a timely opportunity to aware the readers on the potentially wide applications of this surgical approach.
Experimental background: Starr (1943) demonstrated with an experimental study that a severely damaged right ventricle can positively contribute to the pulmonary circulation; Ilbawi (1989) showed that the RV, even with a volume reduced to 30% of normal, has still favourable haemodynamic effects on the pulmonary circulation; and Danton (2001) utilised a malfunctioning right ventricular chamber to manage part of the venous return.
Clinical background: The clinical use of a hypoplastic ventricle to manage part of the venous return has been firstly reported almost simultaneously for both the left [1] and right [2] ventricular hypoplasia.
Pathophysiology: The haemodynamics of one-and-half ventricular repair is characterised by systemic and pulmonary circulations in series, completely separated, with systemic circulation fully supported by a (systemic) ventricle, pulmonary circulation dependent by a superior cavo-pulmonary connection (bidirectional Glenn) and supported by a hypoplastic (pulmonary) ventricle for the IVC return.
Advantages: The advantages of incorporating a hypoplastic ventricle to partly support the pulmonary circulation are the following:
ability to increase the cardiac output;
adaptation to exercise;
maintenance of pulsatile flow in the pulmonary circulation;
flexibility to increased PVR;
circulation at low venous pressure in the IVC system;
capability of a hypoplastic right heart to adequately handle the reduced pre-load.
Indications: One-and-half ventricular repair has been applied to a huge variety of indications, because of right ventricular morphology (small size) or dysfunction, either acute or chronic [2–4]:
pulmonary atresia with intact ventricular septum;
Ebstein's anomaly;
Uhl's anomaly;
atrio-ventricular septal defects (associated with ventricular dominance, tetralogy of Fallot or persistent SVC with unroofed coronary sinus);
double outlet RV (in presence of straddling TV, or uncommitted VSD, or subaortic obstruction);
double discordance.
Right ventricular volume: A decision-making algorithm taking into account the calculated right ventricular volume and the Z value of the TV (=valve size as compared with the ‘normal’ valve size) has been proposed by the group with the largest clinical experience with one-and-half ventricular repair [3].
Expanding indications: Despite words of caution about this approach where a biventricular repair is still feasible [5], the horizon of the one and half ventricular repair is rapidly expanding.
Long-term results: The major concern to the extended application of one and half ventricular repair is the currently limited knowledge of the long-term results, due to the relatively recently utilisation of this surgical approach and to the reduced number of long-term clinical reports.
International registry: The ‘International Registry for One-And-Half Ventricular Repair’ has been announced at the World Congress of Pediatric Cardiology and Cardiac Surgery, Toronto, 2001 (Cardiol Young 2001;11:582; Asian Cardiovasc Thorac Ann 2001;3).
Data collection and analysis of a large number of patients will better clarify the possibilities offered by one and half ventricular repair and therefore will facilitate the future decision-making progress with regard to this promising surgical approach.Antonio F. Corno
Centre Hospitalier Universitaire Vaudois (CHUV), 46 rue du Bugnon, CH-1011 Lausanne, SwitzerlandTel.: +41-21-314-2280; fax: +41-21-314-2278. E-mail address: [email protected].
References
References
- endocardial cushion defects
- fontan procedure
- tetralogy of fallot
- truncus arteriosus
- situs inversus
- ebstein anomaly
- pulmonary artery
- modified blalock-taussig shunt
- balloon dilatation
- lung
- pulmonary circulation
- congenital heart defects
- right ventricle
- ventricular septal defect
- right atrial pressure
- dilatation, pathologic
- follow-up
- preoperative care
- reconstructive surgical procedures
- repeat surgery
- surgical procedures, operative
- heart
- interventricular septum
- atresia
- previous surgery
- oxygen saturation measurement
- venous return
- shunt
- dysplasia
- right ventricular outflow tract
- muscular ventricular septal defect in inlet septum
- cardiac lesion
- central aortopulmonary shunt operation




