-
PDF
- Split View
-
Views
-
Cite
Cite
Chih-Ming Lin, Anna Fen-Yau Li, Li-Hwa Wu, Yu-Chung Wu, Frank Cheau-Feng Lin, Liang-Shun Wang, Adenoid cystic carcinoma of the trachea and bronchus – a clinicopathologic study with DNA flow cytometric analysis and oncogene expression, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 4, October 2002, Pages 621–625, https://doi.org/10.1016/S1010-7940(02)00406-2
Close - Share Icon Share
Abstract
Objective: Adenoid cystic carcinoma (ACC) of the tracheobronchial tree is quite uncommon. The clinicopathologic analysis and the therapeutic outcomes of tracheobronchial ACC have been reported earlier. However, their biological behavior should differ from other tracheal neoplasms. Thus, DNA flow cytometric analysis and biomarkers of p53, HER-2/neu and COX-2 for tracheobronchial ACC were investigated in order to evaluate their clinicopathological significance. Methods: Between 1985 and 1999, nine patients with tracheobronchial ACC were included for the study. All the patients had pathologically confirmed ACC. Five were male and four were female. Eight patients underwent surgical resections. Seven paraffin embedded tumors from six patients were available for DNA flow cytomeric analysis and immunohistochemical staining of p53, HER-2/neu and COX-2. Results: Histologically, nine pathologic specimens from eight surgical patients (including one patient received operation twice) showed one grade I, five grade II and three grade III. The mitotic activity, lymphatic invasion and vascular invasion were more frequent in advanced grading tumor. The higher grade tumors seemed to be associated with a higher synthetic phase fraction (SPF). Immunohistochemically, except for one grade II tumor showing positive expression of HER-2/neu, all the seven tumorous samples revealed negative expressions of p53, COX-2 and HER-2/neu. The patient with positive HER-2/neu tumor had distant metastases 4 years after surgery. Conclusions: Complete surgical resection may provide best survival for tracheobronchial ACC. The DNA ploidy and SPF may correlate with tumor grading or metastasis. The overexpressions of HER-2/neu, p53 and COX-2 may impact the prognosis in patients with stage I non-small cell lung cancer, but did not express difference in our patients.
1 Introduction
Adenoid cystic carcinoma (ACC) of the respiratory tract is rare [1,2]. Although complete surgical resection of the tumor potentially provides a chance of cure, tracheal ACC manifests different clinical behavior, nature history and responses to various treatment modalities from other malignant tracheal tumors [3]. Many studies have been done on tracheobronchial ACCs, but almost all focused on their therapeutic experiences and the clinicopathologic factors affecting the outcome [4,5]. ACC is generally agreed upon as a low-grade malignancy. Several clinicopathologic factors associated with poor prognosis have been recommended, including histological grade, distant metastasis and local recurrence [3,5]. Unfortunately, these prognostic factors were controversial and not conclusive.
Flow cytometric DNA analysis is widely used to characterize the biological behavior of tumors [6,7]. DNA distribution pattern and synthetic phase fraction (SPF) can reflect the malignant potentiality and predict post-treatment relapse and survival for cancerous patients. Nevertheless, little is known about DNA content analysis in tracheobronchial ACC, although ACC of salivary glands has been investigated by Greiner et al. [8].
Our previous study has shown that in stage I lung adenocarcinoma, most of the tumor recurrence developed within 2 years after surgical intervention and was highly associated with overexpression of HER-2/neu oncoprotein [9]. Expression of p53 was thought to be an independent factor to decrease survival of patients with localized adenocarcinoma of lung [10,11]. Additionally, it was suggested that an increase of COX-2 expression in tumor cells may be clinically significant for the prognosis of patients undergoing surgical resection of early-stage adenocarcinomas [12]. Accordingly, no data of biomarker expression in tracheobronchial ACC have been reported previously, and morphologically, ACC is rather similar to adenocarcinoma [13]. We, thus, selectively evaluated the expressions of HER-2/neu, p53 and COX-2 in our surgical specimens of tracheobronchial ACC to compare with stage I adenocarcinoma of lung.
In this study, we used DNA flow cytometric analysis to determine tumorous DNA contents and SPF, and immunohistochemistry to evaluate the expressions of HER-2/neu, p53 and COX-2 in tracheobronchial ACC. In addition to the clinicopathological analysis of our nine patients, the possible relationships between biological markers and clinicopathological factors would be further discussed although only seven surgical specimens obtained from six patients were available for DNA content measurement and immunohistochemical staining.
2 Material and methods
Between January 1985 and December 1999, nine patients with the tracheobronchial ACC were retrospectively collected. Five were male and four were female. Their clinical features were reviewed according to the chart records. The mean age at diagnosis was 50.1 years (range 29–73). The pre-operative work-up included blood cell counts, biochemistry, chest radiography, bronchoscopy with biopsy, computed tomography (CT) of chest, sonogram of neck and abdomen, and radionuclide scanning of whole body bone. Seven tumors were located at the trachea and the other two at bronchus (one at orifice of left upper lobe and the other at truncus intermedius). Surgical resections of the tracheobronchial ACCs were performed for eight patients. The modes of surgical resections consisted of six segmental resections of trachea and two lobectomies. The mean tumor size was 2.1 cm in diameter (range 0.8–3.0). One male patient (Case 6) with tracheal ACC developed a small metastatic nodule at the right lower lung about 4 years after the initial tracheal resection, and wedge resection of the metastatic nodule was performed for diagnosis and treatment. Thus, there were nine surgical specimens. Histologically, all surgical specimens were examined for histological grading, mitotic activity and lymphovascular invasion by the same pathologist, who was unaware of DNA content findings, status of biomarker expression or patient survival. The tumors were further classified into tubular, cribriform and solid subtypes [5]. The histological grades were defined as follows: grade I, tumors with tubular and cribriform subtypes but without solid subtype; grade II, tumors with tubular and cribriform subtypes in which the solid subtype comprised less than 30% of the area; grade III, tumors in which the solid subtype comprised more than 30% of the area. However, only seven paraffin embedded tumors obtained from six patients were available for DNA content measurement and immunohistochemical study.
2.1 DNA flow cytometry [6,7]
DNA histograms can evaluate presynthetic growth phase (G0/G1), synthetic phase (S-phase), and postsynthetic and mitotic growth phase (G2+M). Specimens, 30 μm, from paraffin embedded tumors were taken for DNA flow cytometric analysis. Flow cytometer (Epics Profile II, Coulter Electronics, Hialeh, FL) supplemented with a Cyomics (150 mW) argon ion laser was used for DNA content measurement. The ploid status was defined as diploid (2 N) when G0/G1 peak was superimposed after the addition of peripheral blood lymphocytes to the samples, ranging from 1.9 to 2.1 N. Aneuploid denoted discrete G0/G1 peak(s) ranged outside 1.9–2.1 N.
2.2 Immunohistochemistry
Two paraffin blocks of tumor were prepared for each case. The tissue preparation and staining procedure have been described previously [9]. Briefly, paraffin blocks were sectioned at 4 μm. The wax was melted in a 65°C oven overnight. The slides were deparaffinized in xylene, and xylene was removed subsequently with absolute ethanol before immunostaining. The slides were incubated with monoclonal antibody to HER-2/neu (DAKO A/S, Copenhagen, Denmark), p53 (DAKO) and COX-2 (OXFORD) and then with peroxidase-conjugated immunoglobulins. Diaminobenzidine was used as chromogenic substrate and red or brown color in the cytoplasm or nucleus was identified as positive reaction. Each batch had a positive and a negative control to ensure the quality.
3 Results
The clinicopathological findings and the therapeutic outcomes of nine patients were shown in Table 1 . One patient (Case 8) only received irradiation of 5900 cGy for an unresectable ACC at tracheal carina. Unfortunately, this patient died of pneumonia and sepsis 1 month after radiotherapy. Another patient (Case 5) with tumor growth at truncus intermedius underwent bilobectomy but died of respiratory complication after surgery. Except for these two mortalities, the other seven patients who underwent complete surgical resections were alive till the date of analysis, having been followed for a mean of 6.5 years, ranging from 1.5 to 14.4 years. Two of them developed tumor recurrences. The patient (Case 4) diagnosed to have tracheal ACC at the age of 48 underwent segmental resection of the trachea with postoperative adjuvant radiotherapy of 5400 cGy. The tumor metastasized to T- and L-spines 4 years after the initial operation, and radiotherapy was established for the metastatic bony lesions. Unfortunately, multiple pulmonary metastases developed 3 years later to be administered with a combination chemotherapy of 5-fluorouracil (5-FU) and cisplatin. Till the date of follow-up, she was alive but the disease still progressed slowly. Another patient (Case 6) was found to have a small metastatic nodule in the right lower lung 4 years after the initial tracheal resection, and underwent pulmonary metastatectomy. He survived well without tumor recurrence.
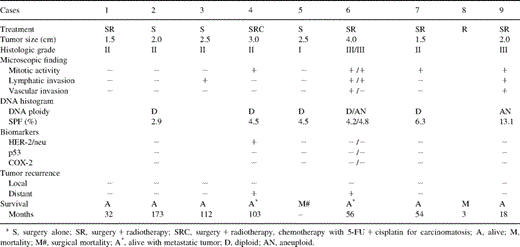
The clinicopathological findings, DNA histogram, biomarkers, and therapeutic outcomes in patients with tracheobronchial ACC
The histological grades of tumors included one grade I, five grade II and three grade III (Table 1). The incidences of positive mitotic activity, evident lymphatic and vascular invasions were 5/9 (55%), 4/9 (44%) and 2/9 (22%), respectively. The tumors with advanced histological grades seemed to associate with higher mitotic activity and evident lymphovascular invasion. All grade III tumors (n=3) were found to have an increase in mitosis as well as lymphovascular invasion. Additionally, distant organ metastasis developed with grade II tumor (Case 4) and grade III tumor (Case 6).
Seven paraffin embedded surgical specimens obtained from six patients were available for DNA flow cytometric analysis and immunohistochemical study. In DNA content measurement, five tumors were diploid and only two were aneuploid (Table 1). The mean SPF of diploid tumors was 4.48% and that of aneuploid tumors was 8.95%. Histologically, both the aneuploid tumors were grade III, and associated with a relatively higher SPF. For the patient (Case 6) with pulmonary metastasis, the primary tracheal ACC was diploid but the metastatic ACC of lung was aneuploid. The patient still lived well at the time of follow-up; no other metastasis was detected for more than 1 year after pulmonary metastatectomy. Immunohistochemically, the expressions of HER-2/neu, p53 and COX-2 were all negative in tumorous and adjacent non-tumorous samples except for one grade II tumor (Case 4) which showed overexpression of HER-2/neu in the cytoplasm of tumor cells (Fig. 1 ). This patient (Case 4) developed multiple distant metastases 4 years later after the initial complete surgical resection.
Immunohistochemical staining of HER-2/neu expression in adenoid cystic carcinoma of trachea (Case 4). (×400).
4 Discussion
Primary ACC of the main-airway occurs with almost equal frequency in both man and woman. The average age at diagnosis of ACC ranges from 45 to 60 years [3–5]. In contrast, squamous cell carcinoma (SCC) of the main-airway occurs with male predominance at quite an advanced age [1,2]. Patients with ACC present with vague symptoms and are often treated for conditions such as asthma or upper respiratory tract infection for months before being diagnosed. In our patients, cough, dyspnea, and stridor or wheezing were the most common clinical features, and the average duration of symptomatic complaints prior to diagnosis was 1.5 months (range 1–3 months). Generally, ACC appears to have a much less progressive course than SCC. In a multicenter restrospective review, Regnard et.al. reported that patients with ACC had symptoms three times as much as that of SCC [14]. Histologically, ACC is a specific variant of adenocarcinoma and occurs most commonly in the salivary glands, and its histological subtypes are classified into tubular, cribriform, and solid ones. The solid subtype of ACC may grow more extensively and invasively than the cribriform and tubular subtypes. Microscopically, we have found that the high grade tumors were frequently associated with increased mitotic activity and evident lymphovascular invasion. Similar to the previous reports, our results indicated that tumor histological grade may correlate with prognosis.
ACC spreads commonly by direct extension, submucous or perineural invasion, or hematogenous metastasis. Lymphatic spread is rather uncommon for tracheal ACC [3]. In our two patients with hematogenous spreading, we did not find locoregional tumor recurrence or lymphatic metastasis till the date of analysis. Although metastases to brain, bone, liver, skin, kidney, abdomen and heart have been reported, pulmonary metastasis is the most common and generally can remain asymptomatic with slow growth [4,15]. However, pulmonary metastatectomy might be suggested for histological diagnosis, or even for tumor burden reduction. It is generally agreed that chemotherapy does not appear to have a role in treatment of ACC of the main-airway or the head and neck [3,16,17]. Only one of our nine patients received a combination chemotherapy of 5-FU and cisplatin for multiple bony and pulmonary metastases. Presently, she is alive and is in rather stable condition, although the metastatic lesion still progresses slowly. Literature review only found a limited number of patients subject to chemotherapy, and no study has directly addressed this particular issue. Thus, the efficacy of chemotherapy for ACC needs further investigation.
Treatments for the primary ACC of the main-airway include surgery or radiotherapy alone, or in combination. Our therapeutic choice was always surgery, as the symptoms of almost all the patients were very serious and no other effective treatment is currently realized. A definite trend towards improving long-term survival with complete rather than incomplete resection has been documented [3,4,14,15]. Nevertheless, we also agree absolutely with the recommendation that safety of the anastomosis is the primary aim in surgery for ACC rather than the histologically examined radicality [4,14]. As in tracheobronchial surgery, there are few data for the extent of resection as ensuring tumor-free margins may endanger the patient. Several previous investigators have reported that histologically free resection margins cannot prevent very late local recurrence of ACC [4]. Furthermore, postoperative irradiation might diminish the rate of local tumor recurrence as even the surgical cut-end margins are not free of cancer cells [1,2,3,18,19,20]. The present series found no local tumor recurrence at the site of anastomosis for those undergoing complete surgical resection with or without postoperative radiotherapy.
In the previous studies of ACC of salivary glands, the vast majority of tumors (77–95%) were diploid [8]. Kino et al. found no difference in survival time between patients with diploid and tetraploid tumors for ACC of salivary glands [21]. In addition, Greiner et al. recommended that SPF analysis is a more useful prognostic predictor than ploidy classification for ACC of salivary gland [8]. Similar to ACC of salivary gland, most (71%) of tracheobronchial ACC were diploid in our patients. However, our results might hint that DNA polidy and SPF could be related to histological grade or even tumor recurrence, although the case number was limited. The SPF of diploid ACC of the main airway was around 4.5% in our series, which was the same as that of salivary gland ACC. Nevertheless, the mean SPF of our two aneuploid ACCs was 9%.
The overexpression of HER-2/neu, p53 and COX-2 in stage I lung adenocarcinoma was 50, 49, and 72%, respectively [9,10,12]. In the present study, however, we did not detect the expressions of p53 or COX-2 in ACC, and only one of seven tumorous samples was found to have increased HER-2/neu immunoreactivity. Interestingly, this patient developed multiple tumor recurrence even after complete surgical resection and local irradiation therapy. Although ACC is a malignant variety of pleomorphic adenoma, our immunohistochemical study demonstrated that ACC of the trachea and bronchus differed remarkably from stage I lung adenocarcinoma in oncoprotein expression.
As a subset of tracheobronchial tumors, ACC has a unique natural history. Complete surgical resection may provide the patients with the best chance of long-term survival although the postoperative irradiation appears to have an effect on local control of the lesion. Our data support the knowledge that certain conventional histological and clinical parameters are useful in predicting the prognosis of ACC. Most ACCs are diploid tumors, but aneuploid ACCs with high SPF might be associated with a higher histological grade. However, no available biomarker could be presently used as a prognostic indicator for tracheobronchial ACCs. A further study is needed to investigate the biological behavior of the ACC.
We would like to thank Ms Li-Ling Yang for her excellent technical assistance. This study is supported by Lite-on Culture Foundation (LCF-R-89-1).
References
- cyclooxygenase-2
- immunohistochemistry
- biological markers
- bronchi
- adenoid cystic carcinoma
- dna
- genes, erbb-2
- neoplasm metastasis
- oncogenes
- paraffin
- ploidies
- receptor, erbb-2
- surgical procedures, operative
- tracheal neoplasms
- neoplasms
- treatment outcome
- tracheobronchial tree
- adenoid cystic carcinoma of trachea
- metastasis, distant
- non-small cell lung cancer stage i
- lymphatic invasion
- excision
- neoplasm grading
- fluid flow




