-
PDF
- Split View
-
Views
-
Cite
Cite
L. Englberger, P. Markart, F.S. Eckstein, F.F. Immer, P.A. Berdat, T.P. Carrel, Aprotinin reduces blood loss in off-pump coronary artery bypass (OPCAB) surgery, European Journal of Cardio-Thoracic Surgery, Volume 22, Issue 4, October 2002, Pages 545–551, https://doi.org/10.1016/S1010-7940(02)00433-5
Close - Share Icon Share
Abstract
Objective: Effects of aprotinin in off-pump coronary artery bypass (OPCAB) surgery have not yet been described. This study analyses hemostasiologic changes and potential benefit in OPCAB patients treated with aprotinin. Methods: In a prospective, double-blind, randomized study 47 patients undergoing OPCAB surgery were investigated. Patients received either aprotinin (2×106 KIU loading dose and 0.5×106 KIU/h during surgery, n=22) or saline solution (control, n=25). Activated clotting time was adjusted to a target of 250 s intraoperatively. Blood samples were taken up to 18 h postoperatively: complete hematologic and hemostasiologic parameters including fibrinopeptide A (FPA) and D-dimer in a subgroup of 31 patients were analyzed. Blood loss, blood transfusion and other clinical data were collected. Results: Both groups showed comparable demographic and intraoperative variables. Forty-one (87%) patients of the whole study group received aspirin within 7 days prior to surgery. Number of grafts per patient were comparable (2.9±1.0 [mean±SD] in the aprotinin group and 2.8±1.2 in control, P=0.83). Blood loss during the first 18 h in intensive care unit was significantly reduced in patients treated with aprotinin (median [25th–75th percentiles]: 500 [395–755] ml vs. 930 [800–1170] ml, P<0.001). Postoperatively only two patients (10%) in the aprotinin group received packed red blood cells, whereas eight (35%) in the control group (P=0.07). Perioperatively FPA levels reflecting thrombin generation were elevated in both groups. The increase in D-dimer levels after surgery was significantly inhibited in the aprotinin group (P<0.001). Early clinical outcome was similar in both groups. Conclusions: Aprotinin significantly reduces blood loss in patients undergoing OPCAB surgery. Inhibition of enhanced fibrinolysis can be observed. FPA generation during and after OPCAB surgery seems not to be influenced by aprotinin.
1 Introduction
Coronary artery bypass grafting (CABG) with cardiopulmonary bypass (CPB) has evolved a safe procedure with low mortality rates in the last decades. However, extracorporeal circulation and its influence on hemostasis, platelet count and function may lead to increased postoperative bleeding, substantial need for foreign blood transfusion and morbidity [1]. In order to reduce CPB associated morbidity, off-pump coronary artery bypass (OPCAB) procedures have gained popularity. Although postoperative bleeding seems to be attenuated by the avoidance of CPB, hemorrhagic complications are not completely eliminated and there is still a need for blood transfusion after OPCAB surgery [2–4].
Aprotinin, a non-specific protease inhibitor, is proven to reduce blood loss and transfusion requirements in on-pump cardiac surgery. In many centers aprotinin is now used on a routine basis for on-pump CABG cases. The aim of this study was to evaluate the hemostatic effects of aprotinin in OPCAB surgery.
2 Material and methods
Forty-nine patients scheduled for elective CABG and supposed to be operated with off-pump techniques were enrolled in this prospective, double-blind, randomized study. Exclusion criteria were the following: previous cardiac surgery, left ventricular ejection fraction <0.35, myocardial infarction <7 days before surgery, neurologic disorders (e.g. cerebrovascular accident), severe pulmonary disorders, renal failure (elevation of creatinine and urea), liver diseases, active inflammatory disease, preoperative coagulopathies, and previous aprotinin therapy. Patients receiving aspirin, other antiplatelet drug or heparin were included in the study. Study protocol was approved by the ethical committee on human research of Berne (6/2000, April 12, 2000). Written informed consent was obtained from all patients. Two patients (4%) were converted to on-pump CABG and were excluded from the study. Patients were randomly allocated to either the aprotinin group (A, n=22) or the control group (C, n=25). In the aprotinin group a loading dose at the beginning of surgery (2×106 KIU=280 mg) was administered followed by continuous infusion of 0.5×106 KIU throughout surgery. In group C the same volume of saline solution was infused. Fifty milliliter bottles with the study drug (0.5×106 KIU=70 mg aprotinin) or with saline solution were numbered continuously. For each number eight bottles with the same content were available. Blinding of the bottles was performed by personal of the hospital pharmacy, not involved otherwise in the study.
2.1 Anesthesia, operation and postoperative care
All patients had premedication with benzodiazepines. General anesthesia was induced and maintained with midazolam, fentanyl and inhalatives (isoflurane or enflurane). Muscle relaxation was achieved with pancuronium bromide. Standard monitoring methods including electrocardiography, radial artery line, central venous catheter, two peripheral venous catheters, urinary catheter and rectal temperature measurement were used in all patients.
All patients were operated on according to a standardized surgical protocol. After full midline sternotomy the left internal mammary artery was harvested in all patients, together with additional graft material (saphenous vein and/or radial artery). Stabilization of the beating heart was established with the Octopus® tissue stabilizer (Medtronic Inc., Minneapolis, MN, USA).
Heparin IV was administered after harvesting the internal mammary artery to achieve systemic anticoagulation during surgery by an initial dose between 70 and 100 units/kg bodyweight. Activated clotting time (ACT) was adjusted to a target of 250 s intraoperatively. ACT was measured using a kaolin-activated system (Automated coagulation timer ACT II, Medtronic HemoTec Inc., Englewood, FL, USA). Standard use of protamine after the procedure was not intended. It was only given if clinically increased bleeding in the operating field was observed and/or if the ACT value was still over 200 s at the end of surgery. A cell-saving device was not used. Intraoperatively no blood was retransfused. Transfusion of packed red blood cells was performed if the hematocrit was less than 23%. Before chest closure, mediastinal and pleural drains were inserted and low-grade suction (20 cm H2O) was performed. Blood loss was recorded at arrival in the intensive care unit (ICU), 2, 6, 12 and 18 h after surgery.
In ICU the patients were treated according to routine protocol. Up to 12 h after surgery shed mediastinal blood was retransfused (Autotransfusion Reservoir, Jostra Medizintechnik, Hirrlingen, Germany) if necessary (hemoglobin <90 g/l and total drainage volume exceeded 250 ml). A red blood cell transfusion was administered when hemoglobin decreased to less than 85 g/l. Transfusion of fresh frozen plasma was indicated to correct suspected deficiency of coagulation factors when drain production was increased (>200 ml/h). Prophylactic measures were started at the first postoperative day with orally given aspirin (100 mg once per day) and subcutaneous injection of low-molecular-weight heparin (weight-adapted dosage once per day).
2.2 Laboratory analyses
Perioperatively, routine hematologic (hemoglobin, hematocrit, platelet count) and hematochemical parameters such as creatinine, creatine phosphokinase (CK) including its iso-enzyme MB and Troponin I were collected in all patients and the samples were analyzed by the central laboratory of the hospital for routine analysis.
In a subgroup of patients (n=16 in group A, n=15 in the control group) arterial blood samples were collected at six different time points to determine fibrinopeptide A (FPA) and D-dimer: preoperatively, after induction of anesthesia (baseline); during distal anastomoses; at the end of surgery; 2, 6 and 18 h postoperatively. To determine parameters of coagulation and fibrinolysis blood was used treated with CTADPPACK anticoagulant. Immediately the corpuscular content was separated from the fluid phase by centrifugation at 2000g for 15 min at 6°C. Plasma was aliquoted and stored at −70°C before being assayed. FPA was measured using a radioimmunoassay (RIA), (IMCO Corporation Ltd AB, Stockholm, Sweden). D-dimer (Dimertest®, Agen Biomedical, Acacia Ridge, Australia) which reflects the fibrinolytic status was measured using enzyme-linked immunosorbent assay (ELISA) techniques. All samples were assayed in duplicate and the mean value was retained.
2.3 Statistical analysis
Statistical analysis was carried out using standard software (StatView 5.0.1, SAS Institute Inc., Cary, NC, USA). Data are presented as median [25th percentile; 75th percentile] or as mean±SD if appropriate. Normally distributed data were analyzed with two-tailed unpaired t-test. A chi-square analysis or Fisher's exact test was used for categorial data. Non-parametric evaluation was performed for variables not normally distributed (Mann–Whitney U test). Laboratory results were tested within the groups to detect time interactions (Friedman test). If statistically significant changes were seen, signed-rank testing was post hoc used to locate the differences. Differences between the groups were analyzed using two-group non-parametrical testing (Mann–Whitney U). For repeatedly measured variables additional global testing (analysis of variance (ANOVA)) for both groups was performed. For multiple testing Bonferroni correction was performed. A P value less then 0.05 was considered statistically significant.
3 Results
Both groups showed comparable demographic and intraoperative variables (Table 1 ). No statistically significant differences were noted between the groups with respect to mean age, gender, body mass index, left ventricular function, mean duration of the operation and number of grafts per patient. Forty-one (87%) patients of the whole study group received aspirin within 7 days prior to surgery (Table 1).
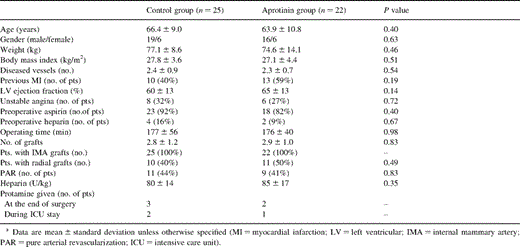
Two patients (one in each group) required reexploration because of bleeding. In both patients a surgical cause of bleeding was detected. One patient of the control group had signs of myocardial ischemia with hemodynamic instability after surgery and was transferred back to the operating room. Spasm of the radial graft to the obtuse marginal was detected and a venous graft to the marginal branch was performed with CPB. The postoperative variables of these three patients (laboratory results, blood loss and transfusion-requirements) were not included in the analysis.
Heparin dose did not differ between the groups (Table 1). In both groups ACT levels were measured in the adjusted range after heparin (230 [210–295] s in the aprotinin group vs. 243 [203–284] s in control, P=0.98) and returned to baseline values 2 h postoperatively (110 [98–131] s vs. 103 [86–115] s, P=0.16). At the end of surgery ACT values were 197 [168–243] s and 191 [154–218] s, respectively (P=0.30). Protamine was administrated only in a minority of patients (Table 1).
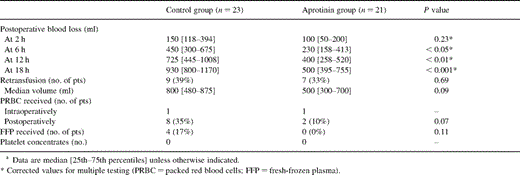
Blood loss during the first 2 h in ICU did not differ significantly between the groups whereas at the following time points patients treated with aprotinin had highly significant less blood loss than the control group (500 [395–755] ml vs. 930 [800–1170] ml, P<0.001). Postoperatively only two patients (10%) in the aprotinin group received packed red blood cells, whereas eight (35%) received in the control group (P=0.07). Only four patients received fresh-frozen plasma, all in the control group (P=0.11). No patient of either group had transfusion of platelet concentrates (Table 2 ).
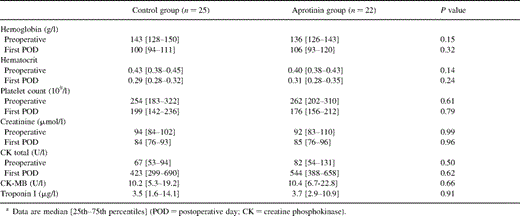
Routine hematological and hematochemical data compared preoperatively and at the first postoperative day showed no significant differences between the groups (Table 3 ).
Early clinical outcome was similar in both groups. The incidence of adverse events during hospitalization was comparable in both groups (Table 4 ). The patient who required reoperation because of a radial artery spasm suffered from perioperative myocardial infarction, a neurological impairment, a prolonged ventilation time and a transient renal failure with the need for dialysis.

3.1 Fibrinopeptide A and D-dimer
As indicator of thrombin formation, the plasma concentration of FPA was elevated perioperatively with peak values at the end of surgery (21.2 [16.9–28.6] ng/ml in the aprotinin group and 25.6 [21.2–28.8] ng/ml in control). Six hours postoperatively median values were higher in the control group (7.4 [5.2–10.6] ng/ml vs. 18.8 [10.6–25.3] ng/ml, P<0.01). Nevertheless, global testing showed no significant treatment interaction between the groups (P=0.61). Furthermore, eliminating values of patients who had postoperative retransfusion of mediastinal shed blood (7/16 in the aprotinin subgroup and 7/15 in control), the difference was minimized (9.0 [7.6–13.2] ng/ml vs. 11.1 [9.8–18.0] ng/ml, P=0.30). Overall, elevated median values of FPA at the end of surgery compared to baseline in both groups demonstrate reasonable generation of thrombin. Significant influence of aprotinin was not detected (Fig. 1 ).
Fibrinopeptide A (FPA). Data are presented as median. Error bars indicate 25th and 75th percentiles. +, significant higher values compared to baseline (P<0.05). *, significant intergroup difference (P<0.01) (H=hours postoperatively).
Preoperative median levels of D-dimer were comparable in both groups. Postoperatively, both groups showed significantly elevated levels of D-dimer compared to baseline. At the end of surgery and at the following sampling points up to 18 h postoperatively a highly significant difference between the groups was detected. The increase in D-dimer levels after surgery was significantly inhibited in the aprotinin group (P<0.001), (Fig. 2 ). Significant intergroup differences persisted, if the patients with postoperative retransfusion of mediastinal shed blood were excluded from analysis.
Levels of D-dimer. Data are presented as median. Error bars indicate 25th and 75th percentiles. Higher values compared to baseline in both groups (+P<0.05; ++P<0.01). Significant intergroup differences (*P<0.05; **P<0.01; ***P<0.001) (H=hours postoperatively).
4 Discussion
It is well recognized that aprotinin reduces both, blood loss and the need for foreign blood-product transfusion following CABG with CPB [5,6]. During the recent years an increasing number of OPCAB procedures have been performed. However, beneficial effects of aprotinin have not yet been tested in this setting. Besides, its hemostatic effects in cardiac surgery the efficacy of aprotinin has been demonstrated in other surgical disciplines (e.g. liver, thoracic and major orthopedic surgery) [7–9] but not in open abdominal aneurysm repair [10]. Some previous reports have demonstrated decreased perioperative bleeding and reduced use of foreign blood products in OPCAB surgery compared to CABG with extracorporeal circulation; nevertheless, postoperative blood loss following OPCAB surgery cannot be neglected [2–4]. Our own experience demonstrates an increasing proportion of patients operated without cessation of antiplatelet therapy. Additionally it has been described, that bleeding complications occurring may be controlled and/or compensated by the routine use of aprotinin [11,12]. In this prospective, double-blind, randomized study we evaluated the effectiveness of aprotinin in OPCAB surgery.
The main end-point ‘postoperative blood loss’ showed a highly significant effect of aprotinin with reduced blood loss in the treatment group. Consecutively, less patients in the aprotinin group received foreign blood products (35% vs. 10%) in the postoperative course. However, the difference between the two groups did not reach statistical significance (P=0.07) in our limited number of study patients, even though it seems to be clinically relevant. The proportion of patients who received postoperative retransfusion were comparable, but not the retransfused volume, which tends to be lower in patients treated with aprotinin (Table 2). Overall, our data demonstrate relevant clinical efficacy of aprotinin in OPCAB patients.
In order to investigate the effects of aprotinin on coagulation and the fibrinolytic system in OPCAB patients, two important plasmatic markers (FPA and D-dimer, respectively) were determined in a subgroup of 31 patients. The coagulation product FPA was elevated perioperatively in both groups; this reflects activation of thrombin which has been described also otherwise in major surgery [13] and in OPCAB procedures [14]. Intraoperative anticoagulation was performed with weight-adjusted heparin (70–100 U/kg bodyweight in all patients) and ACT value of 250 s was targeted. Reversal with protamine was performed only in a minority of patients. No thrombembolic complication occurred in our study population. So far concerns raised about procoagulant activity after OPCAB surgery [14] can be supported only in biochemical terms. In addition, 100 U/kg heparin seems to become the standard in OPCAB procedures, and recent investigations did not show a higher incidence of thrombotic complications in OPCAB surgery [15]. Nevertheless, aprotinin had no significant impact on the median levels of FPA in our study cohort, despite others having hypothesized anticoagulative/antithrombotic effects of this drug [16–18]. Detected higher values of FPA in the control group 6 h postoperatively seem to be confounded by retransfused mediastinal shed blood in some patients during the first postoperative hours. Retransfused mediastinal shed blood may be strongly activated and contains some coagulation products in a high concentration [19]. On the other hand, concerns regarding thrombotic complications with the use of aprotinin have been raised. This has led to the recommendation of higher ACT levels during surgery if aprotinin is used [20]. However, this cannot be disproved by studies with small sample sizes, although these concerns may be partially explained by the use of different types of activators for ACT measurement [20]. In our study ACT levels were determined using a kaolin-activated system that in contrast to celite-activation does not produce prolonged ACT values in the presence of aprotinin [16,21].
Looking at the fibrinolytic system we found significantly higher values of D-dimer in the control group. Intergroup difference was already seen at the end of surgery and marked in the postoperative hours. This underlines that improved hemostasis is achieved at least partly by the antifibrinolytic action of aprotinin.
Although antifibrinolytics have been used routinely in cardiac surgery with extracorporeal circulation this measure is not yet established in OPCAB surgery. Recently, Casati et al. [22] demonstrated the effectiveness of the synthetic antifibrinolytic drug tranexamic acid in OPCAB patients. Compared to synthetic substances, the non-specific protease inhibitor aprotinin, isolated from bovine lung tissue, seems to be important not only as an antifibrinolytic agent but also as a modulator of the endothelial function [23] and the inflammatory process [24] and may have superior effects than other drugs [25].
More than 80% of the patients in our study received aspirin within 7 days prior to surgery. We conclude that aprotinin should be considered as adjunct in OPCAB surgery especially in these patients with an increased risk of perioperative bleeding.
We thank Professor Dr A. Haeberli, Mrs T. Pham, Mrs M. Stutz (Thrombosis Research Laboratory at the University Hospital Berne) for laboratory help, Mrs D. Pfiffner for statistical support (Department of Cardiology at the University Hospital Berne) and Professor Dr U. Nydegger.




