-
PDF
- Split View
-
Views
-
Cite
Cite
Christophoros Kotoulas, George Lazopoulos, Theodoros Karaiskos, Pericles Tomos, Marios Konstantinou, George Papamichalis, Dimitra Politi, Achilles Lioulias, Prognostic significance of pleural lavage cytology after resection for non-small cell lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 330–334, https://doi.org/10.1016/S1010-7940(01)00718-7
Close - Share Icon Share
Abstract
Objective: In the staging of lung cancer, pleural effusion that is malignant on cytologic examination is regarded as T4 disease, and curative resection cannot be performed. We conducted this study to determine whether cancer cells can be present in the pleural cavity with no pleural effusion, to investigate the factors contributing to that occurrence, and to evaluate its prognostic significance. Methods: Eighty-five patients (77 males, eight females) with a median age 60.1 ± 7.9 years (31–74 years) underwent a major lung resection, due to lung cancer in our department. From January 1998 to December 1999, 30 pneumonectomies, seven bilobectomies, 46 lobectomies and two wedge-resections were performed. Chest wall resection was performed in four patients. After performing a posterolateral thoracotomy and lung resection with extended mediastinal lymph node dissection, the pleural cavity was filled with 1 l physiologic saline solution (PSS) and the fluid was shaken. The lavage fluid was suctioned off (S1). Immediately after the lavage, the pleural cavity was refilled with 3 l PSS. The surgeon washed out the pleural cavity by hand for 1 min and the fluid was suctioned off. Finally, the pleural cavity was refilled with 1 l PSS and a new lavage fluid was suctioned off (S2). A cytologic examination was carried out for each sample. Results: The pathology report showed 39 adenocarcinomas, 33 squamous-cell, two adenosquamous, four large-cell, two neuroendocrine and five undifferentiated carcinomas. S1 was positive in eight patients (9.4%), while S2 was positive in four patients (4.7%). The correlation of positive pleural lavage and infiltrated lymph nodes demonstrated a statistically significant relation between presence of N2 disease and positive S2 sample (P = 0.049). No significant correlation existed between positive lavage sample (S1 or S2) and TNM stage, level of T, extent of tumor invasion, kind of operation, histological type or differentiation of the cancer (Chi square test). The mean follow-up is 11.3 ± 6.2 months (4–22 months). There are 78 patients alive. A significance difference in survival was identified in-patients with positive S1 (P = 0.0081), and positive S2 (P = 0.0251) (Kaplan–Meier). Conclusion: The cytologic results of lavage were positive for malignant cells in eight of 85 patients (9.4%). The existence of cancer cells in the pleural cavity can be the result of their exfoliation or surgical manipulations. The mechanical irrigation subdivides the percentage of positive samples. Our study supports that the positive findings on pleural lavage cytology is an essential prognostic factor.
1 Introduction
In the TNM staging of lung cancer, the presence of malignant pleural effusion is regarded as T4 [1]. Resulting from this, patients cannot undergo a curative resection and prognosis is very poor. Researchers have demonstrated cases, in which, although preoperative work-up has been negative to malignant pleural effusion, intraoperatively, presence of pleural malignant cells has been observed. This finding should be considered as T4, according to some researchers, while others believe the positive cytological findings of pleural lavage means only a greater possibility of recurrence in the ipsilateral pleural cavity or pericardium than a negative finding [2,3].
We conducted this study to determine whether cancer cells can be present in the pleural cavity with no pleural effusion, to investigate the factors contributing to that occurrence, and to evaluate its prognostic significance.
2 Material and methods
2.1 Patients
During the period from January 1998 to December 1999, 250 patients underwent major lung resection due to lung cancer. As an experimental evaluation, 85 patients – 77 males and eight females, with median age 60.1 ± 7.9 years (31–74 years) – were randomly selected and underwent pleural lavage right after lung resection, in order to determine the presence of malignant cells in the pleural cavity. No patient received preoperatively chemo- or radiotherapy. Preoperative evaluation included a detailed history and physical examination, biochemical profile, chest X-ray examination, bronchoscopy, computed tomography of the chest, the abdomen and the brain. Routine cervical or anterior mediastinoscopy was not performed in the presence of a negative mediastinal window shown by tomographic examination. No patient had distant metastasis. No patient had pleural effusion (malignant or not) at the preoperative imaging control as well as intraoperatively. No patient underwent FNA preoperatively. Thirty pneumonectomies, seven bilobectomies, 46 lobectomies and two wedge resections were performed. En bloc resection of lung and chest wall was performed in four patients. Postoperative mortality was null.
2.2 Methods
After a posterolateral thoracotomy and lung resection with extended mediastinal lymph node dissection, the pleural cavity was filled with 1 l physiologic saline solution (PSS) and the fluid was shaken. The lavage fluid was suctioned off (S1). Immediately after the lavage, the pleural cavity was refilled with 3 l PSS. The surgeon washed out the pleural cavity by hand for 1 min and the fluid was suctioned off. Finally, the pleural cavity was refilled with 1 l PSS and a new lavage fluid was suctioned off (S2).
A cytologic examination was carried out for each sample. After microscopic examination, the results of the cytologic examination were divided into three categories: ‘negative’, ‘suspected’ and ‘positive’. Intraoperatively, complete mediastinal lymph node dissection was performed and the lymph nodes were numbered according Naruke's nomenclature [4]. The histologic type of the tumors was determined by applying the WHO classification [5]. The primary tumor and lymph nodes status were classified according to the international staging system reported by Mountain [1].
Fisher's exact test was used when appropriate; otherwise the chi-square test of independence was employed. Survival curves were calculated to the method of Kaplan–Meier and the log-rank test was used to compare the survival curves [6]. The survival was calculated without censored data. P < 0.05 was accepted as the significance limit.
3 Results
S1 was positive in eight patients (9.4%), while S2 was positive in four patients (4.7%). S1 and S2 were negative for the rest 77 patients. None of the samples was classified as ‘suspected’. Patients characteristics with at least one positive sample are presented at Table 1 .
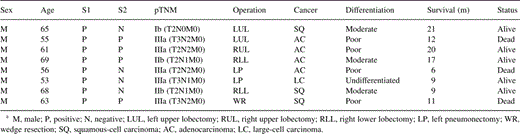
The pathology report showed 39 adenocarcinomas, 33 squamous-cell, two adenosquamous, four large-cell, two neuroendocrine and five undifferentiated carcinomas. The cancer was well differentiated in seven patients, moderate in 26 patients, poor in 46 patients and undifferentiated in six patients.
We divided the 85 patients in four categories according to the extent of tumor invasion: R0 with no invasion of visceral pleura, R1 with invasion of visceral pleura, R2 with disruption of visceral pleura, and R3 with invasion of chest wall. Frequencies of positive cytological findings in each level of R are presented in Table 2 .

The pTNM was Ia in five cases, Ib in 16 cases, IIa in five cases, IIb in 29 cases and IIIa in 30 cases. There was no patient in stage Ia and IIa with positive lavage. Frequencies of positive cytological findings in stage Ib, IIb, IIIa were 6.25, 6.89, 16.67%, respectively. It is observed that the rate increases accordingly to the disease stage. Frequencies of positive cytological findings in each level of T or N are presented in Tables 3 and 4 .


3.1 Statistics
The correlation of positive pleural lavage S1 or S2 and infiltrated lymph nodes N1 or N2 demonstrated a statistically significant relation between presence of N2 disease and positive S2 sample (P = 0.049). Marginal statistical relation was observed between the existence of R2 tumor and positive samples (P = 0.080), as well as between stage IIIa tumors and positive samples (P = 0.085). There was no statistical correlation between level of T, TNM-stage, histological type and differentiation of the tumor, and kind of operation with positive S1 or S2 samples. Marginal statistical difference revealed between R1–R3 and N0–N2 with positive S1 or S2 samples (P = 0.177 - P = 0.055 and P = 0.077 - P = 0.052, respectively).
3.2 Survival
Patients with IIIA stage, as well as patients with chest wall invasion and patients with at least one positive lavage sample undertook chemotherapy and/or radiotherapy. The mean follow-up is 11.3 ± 6.2 months (4–22 months). There are 78 patients alive. Three patients died due to distant metastases, while four others died due to locoregional recurrence. Table 5 presents the characteristics of the seven patients who died. A significance difference in survival was identified in-patients with positive S1 (P = 0.0081) and positive S2 (P = 0.0251), as we can see in Figs. 1 and 2 .
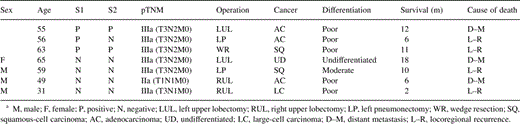
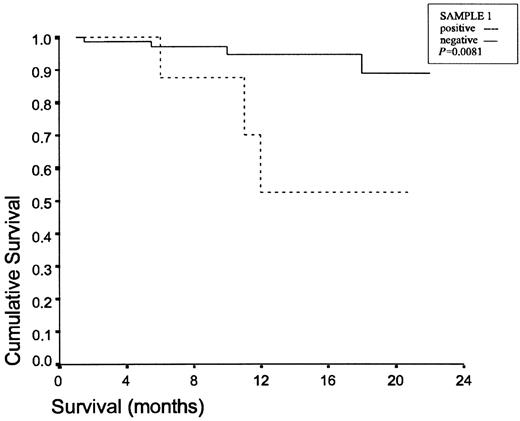
Survival curves for the positive S1 lavage group and the negative S1 lavage group.
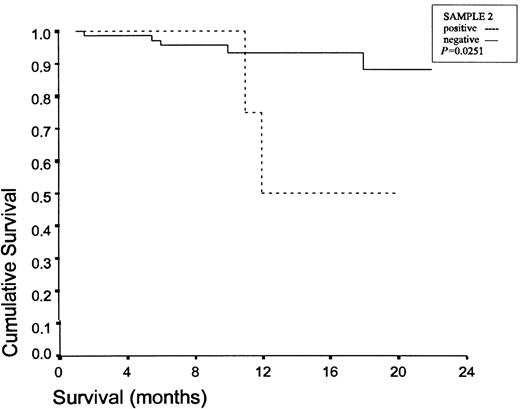
Survival curves for the positive S2 lavage group and the negative S2 lavage group.
4 Discussion
In cases of lung cancer, the pleural lavage cytology, either post-thoracotomy time, or post-lung resection time, has been the objective of a few studies in the past. The percentage of positive pleural lavage cytology ranged from 3.7 to 38.6%. Spjut et al. were the first to observe in 1958 that an average of 32.7% (16/49) had malignant pleural lavage after major lung resection for lung cancer [7]. Eagan et al. observed similar results in an average of 8.9% (12/135) [8]. In 1989, Kondo et al. performing pleural lavage before lung resection in cases with and without pleural effusion, concluded that 9% (42/467) of the cases depicted malignant cells. This was for the first time associated with patient survival and constituted as a prognostic factor [9]. In 1991, Okumura et al. performed the pleural lavage before and after lung resection, finding out that 14% (23/164) had at least one malignant sample. This finding was correlated to a greater possibility of recurrence in the ipsilateral pleural cavity or pericardium than a negative finding [3]. In 1997, Buhr et al. proved also the value of pleural lavage as a prognostic factor, performing the pleural lavage in patients with lung cancer, reported a positive result in 38.6% (132/342) before resection and in 28.95% (99/342) after it, while 2.6% had only a positive lavage after resection [2]. Finally, in 1999, Okada et al. in a series of 482 patients observed that in an average of 3.7% (18 patients) despite the absence of pleural effusion and any surgical manipulation, malignant cells were detected during pleural lavage only in cases of adenocarcinoma. He emphasizes the prognostic value, as well as the need for restaging in such cases [10]. Positive pleural lavage cytology was observed in 9.4% (8/85) of our cases. This supports the results of previous mentioned researchers.
The first main question however still remains; as to which is the cause of presence of cancer-cells in the pleural cavity. One could assume that only surgical manipulations lead to it. Okada et al. showed that the detection of tumor cells in pleural lavage fluid before resection, in cases of lung cancer without pleural effusion, proves that tumor cells have spread in the pleural cavity, even in the early stages of lung cancer, the incidence of which had significant correlations with histology, pleural status, lymphatic permeation and vascular involvement [10]. They support that it is the result of the combination of impairment of lymphatic drainage through the intrapulmonary lymphatic channel to the mediastinal nodes and the result of exfoliation of cancer cells [10,11].
We studied exclusively only the possibility of malignant cell presence in pleural lavage after major lung resection in patients without evidence of pleural effusion or FNA preoperatively. Despite the small patient sample of our study, the fact that only three surgeons performed the operations adds value to it, because of the limited variety of operative manoeuvres. No patient with Ia and IIa disease had positive pleural lavage, while we observed that the positive lavage cytology rate was increased accordingly to the disease stage. Our statistical study revealed the correlation between the presence of N2-disease and positive S2 sample, as well as the marginal difference between R1–R3 and N0–N2 disease. Therefore, we may assume that the mediastinal lymphatic spread can be a sufficient cause for the presence of malignant cells in the pleural cavity. It should be noted that all our patients underwent extended mediastinal lymph node dissection. So, despite the possibility of tumor-cells pre-existence in the pleural cavity, surgical manipulations could be another reason for the positive pleural cytology.
Besides, Buhr et al. showed that 2.6% of the patients presented positive pleural lavage cytology only in the post-lung resection time, while it was negative in the post-thoracotomy time. They supported that it was the result of the adhesion of the lung with the parietal pleura in most of these cases.
There is one more main question; as to what extent does positive pleural lavage cytology influence patients survival. Some researchers support that the positive cytologic findings in pleural lavage fluid indicate T4 spread of the lung cancer, so the disease should be restaged, while others support that only the local recurrence rate increases. We found that locoregional recurrence is no more common in patients with a positive pleural lavage than in those with a negative result. Our survival study certifies the significant difference between patients with positive S1 or S2 sample and the rest of these. So, we may support that the positive pleural lavage cytology in the post-lung resection time is a T4 element and it upgrades the disease to stage IIIB.
A major point resulting from our study is the subdivision of malignant cells concentration between S1 and S2 sample. We believe that mechanical irrigation has an important role in disease prognosis and may increase the long-term survival.
In conclusion we should point out that pleural lavage after lung resection remains an important prognostic factor, as it restages the disease in an average of 9.4% of patients with subclinical presence of cancer cells in pleural cavity, decreasing the long term survival rates. We support that the main causes of positive lavage cytology are either the exfoliation of cancer-cells into the pleural cavity from infiltrated mediastinal lymph nodes, or the spreading of the cancer-cells of infiltrated mediastinal lymph nodes due to surgical manipulations during the mediastinal dissection. Finally, this study showed that mechanical irrigation after lung resection may subdivide the percentage of subclinical malignant pleural effusions.
DrD.VanRaemdonck (Leuven,Belgium): Do you have the possibility of looking at this pleural lavage fluid at the start of thoracotomy, by sending it to your pathologists, and, if so, and they give you a positive result, would you then close the patient and not continue with the resection? You have well demonstrated that the survival is very poor, so why resect the lung in these patients?
DrKotoulas: This is a study for pleural lavage after major lung resection. If we had a patient with a positive pleural lavage before the resection, we should not operate him.
DrVanRaemdonck: Have you asked your cytologists to see if it is possible to have the results during the thoracotomy?
DrKotoulas: No, we don't have this possibility in our hospital.
DrVanRaemdonck: They say they can do it.
DrH.-B.Ris (Leuven,Belgium): You have shown indeed that there is a significant difference in survival between lavage-positive and lavage-negative patients, but you also claimed that you have a significant correlation between positive lavage and the stage of primary tumor. Could it be that in fact this survival difference is more related to the stage of the tumor than to the findings of lavage per se?
DrO.Maiwand (London,UK): Your paper suggests pleural washing helped survival, but your survival rate has not shown different results compared to standard figures.
The second comment is, would breaking the lymph nodes during dissection let some malignant cells float in the pleural cavity.
DrKotoulas: We performed complete mediastinal lymph node dissection in all patients, paying attention to resect the lymph nodes without ruptures or partial removing.
DrT.Dosios (Athens,Greece): If you have a positive pleural lavage preoperatively, I think you have to put the patient on the T4 status. If you have a positive lavage postoperatively, you should characterize the patient as having an incomplete resection. In such a case you should not increase the T status, for example, from T3 or T2 to T4. Regarding the comment that if you have a positive preoperative lavage, you should not resect the tumor, I would disagree. I would prefer to resect the primary tumor and put the patient in chemotherapy. Apparently we need a prospective study to see what is the influence of preoperative lavage on long-term survival.
DrM.Perelman (Moscow,Russia): I fully agree with the role of good mechanical irrigation of the pleural cavity after resection. For many years we used low-frequency ultrasound for the good mechanical debridement of the pleural cavity. The debridement of the pleural cavity with low-frequency ultrasound for 5, 10 min is a very good method for killing all cancer cells and for good debridement after lung resection.
DrRis: I would think that it's premature to say that a positive lavage will not clinically evidence malignant effusion corresponds to a T4 tumor actually but further investigations are indeed necessary.
References
- second heart sound, s2
- pleural effusion
- cancer
- cytology
- adenocarcinoma
- non-small-cell lung carcinoma
- follow-up
- irrigation
- neurosecretory systems
- pneumonectomy
- surgical procedures, operative
- lymph nodes
- mediastinum
- pathology
- pleura
- patient prognosis
- lung volume reduction
- lymph node dissection
- lung cancer
- undifferentiated carcinoma
- peeling of skin
- tnm lung tumor staging
- lobectomy
- posterolateral thoracotomy
- pleural lavage
- prognostic factors
- squamous epithelial cell
- tumor cell invasion
- saline solutions
- tumor cells, malignant




