-
PDF
- Split View
-
Views
-
Cite
Cite
G.P. Eising, M. Niemeyer, Th. Günther, P. Tassani, M. Pfauder, H. Schad, R. Lange, Does a hyperoncotic cardiopulmonary bypass prime affect extravascular lung water and cardiopulmonary function in patients undergoing coronary artery bypass surgery?, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 282–289, https://doi.org/10.1016/S1010-7940(01)00804-1
Close - Share Icon Share
Abstract
Objective: Different types of colloidal priming for cardiopulmonary bypass (CPB) have been used to reduce fluid load and to avoid the fall of plasma colloid osmotic pressure (COP) that leads to edema formation and consequently can cause organ dysfunction. The discussion about the optimal priming composition, however, is still controversial. We investigated the effect of a hyperoncotic CPB-prime with hydroxyethyl starch (HES) 10% (200;0.5) on extravascular lung water (EVLW) and post-pump cardiac and pulmonary functions. Methods: In 20 randomized patients undergoing elective coronary artery bypass graft surgery (CABG), a colloid prime (COP: 48 mmHg, HES-group, n = 10) and a crystalloid prime (Ringer's lactate, crystalloid group, n = 10) of equal volume were compared with respect to the effects on cardiopulmonary function. Cardiac index (CI), mean arterial pressure (MAP), pulmonary capillary wedge pressure (PCWP), systemic vascular resistance index (SVRI), pulmonary artery pressure (PAP), pulmonary vascular resistance index (PVRI), alveolo-arterial oxygen difference (AaDO2), pulmonary shunt fraction (Qs/QT), EVLW (double-indicator dilution technique with ice-cold indocyanine green), COP, fluid balance and body weight were evaluated peri-operatively. Results: Pre-operative demographic and clinical data, CPB-time, cross-clamp time and the number of anastomoses were comparable for both groups. During CPB, COP was reduced by 20% in the HES-group (18.9 ± 3.7 vs. 23.7 ± 2.2 mmHg, P < 0.05) while it was reduced by more than 50% of the pre-CPB value (9.8 ± 2.0 vs. 21.4 ± 2.1 mmHg, P < 0.05) in the crystalloid group (P < 0.05 HES- vs. crystalloid group). Post-CPB EVLW was unchanged in the HES-group but it was elevated by 22% in the crystalloid group (P < 0.05 HES- vs. crystalloid group), CI was higher in the HES-group (3.4 ± 0.3 vs. 2.7 ± 0.51/min, P < 0.05). Fluid balance was less in the HES-group (813 < 619 vs. 2143 < 538, P < 0.05). Post-operative weight gain could be prevented in the HES-group but not in the crystalloid group (1.5 ± 1.2 vs. -0.3 ± 1.5, P < 0.05). No significant differences were seen for MAP, PAP, PCWP, SVRI, PVRI, AaDO2 and (Qs/QT) between the two groups at any time. Conclusions: Hyperoncotic CPB-prime using HES 10% improves CI and prevents EVLW accumulation in the early post-pump period, while pulmonary function is unchanged. This effect can be of benefit especially in patients with congestive heart failure.
1 Introduction
The impairment of cardiac and pulmonary functions following cardiopulmonary bypass (CPB) in cardiac surgery is a well-known phenomenon. Hemodilution by the crystalloid priming of the extracorporeal circulation (ECC) and in consequence the fall of colloid osmotic pressure (COP) with the initiation of CPB was shown to play a key role for post-pump organ dysfunction [1–5]. Although much effort has been expended in the last 25 years, the discussion of the optimal priming composition for the ECC circuit to avoid or at least to ameliorate post-pump organ dysfunction is still controversial and was recently summarized by Boldt [6]. Factors contributing to cardiopulmonary dysfunction following CPB include rise in extravascular lung water (EVLW) [1,2] and myocardial edema formation [7–9]. In general, edema formation results from an imbalance of microvascular net filtration and removal of interstitial fluid by the lymphatics. An important determinant of fluid filtration is the COP of plasma, which counteracts the microvascular blood pressure. A decrease in COP increases effective filtration pressure and thus microvascular filtration. COP is reduced during CPB, if the ECC circuit is primed by crystalloidal fluids and if crystalloidal cardioplegic solutions are used. This decrease in COP and the resulting extravasation of fluid is to attenuate by hyperoncotic priming of the ECC.
Therefore we undertook the following study to evaluate whether a hyperoncotic ECC prime solution beneficially affects EVLW, pulmonary function (shunt flow, oxygenation) and cardiac output in patients undergoing elective coronary artery bypass graft surgery (CABG).
2 Materials and methods
After the study protocol was approved by the ethical committee of the Technical University of Munich and written informed consent from each patient was obtained, 20 consecutive patients scheduled for elective CABG were investigated. Patients were randomly allocated to one of the two groups. Ten patients received a hyperoncotic CPB prime with a colloid-osmotic pressure of 48 mmHg (hydroxyethyl starch (HES) group), as determined by a membrane osmometer (see Section 2.4). The priming solution consisted of HES 10% (200;0.5) 1100 ml (Fresenius, Bad Homburg, Germany), mannitol 20% 3 ml/kg BW, sodium bicarbonate 4.2% 5 ml/kg BW, Inzolen 20 ml (Köhler, Alsbach, Germany) (≅5.55 mmol K+) and heparin 5000 IU (Ratiopharm, Ulm, Germany) (Table 1 ). The other patients (crystalloid group, n = 10) served as control patients and instead of HES 10% Ringer's lactate of equal volume was used (Table 1). Patients showing the following characteristics were not included in the study: age ≫75 years; body weight ±30% of ideal body weight; left ventricular ejection fraction ≪40%; hemodynamic instability or emergency operations; additional valvular diseases; complete bundle branch block; third degree AV-block; impaired renal function (creatinine ≫1.3 mg%); hematocrit ≪30%.

Pre-medication was performed by i.m. application of flunitrazepam 2 mg (Rohypnol®, Roche, Grenzach, Germany), morphine-hydrochloride 10 mg (Merck, Darmstadt, Germany), and atropine 0.5 mg (Braun, Melsungen, Germany). Induction of anesthesia was performed with 1 μg/kg sufentanil, 0.04 mg/kg midazolam and 0.1 mg/kg pancuronium. After orotracheal intubation, mechanical ventilation with 100% oxygen was provided. Tidal-volume was adjusted to achieve normocapnia, controlled by blood-gas analysis. A ‘loading dose’ of 6 μg/kg sufentanil and 0.3 mg/kg midazolam was given before sternotomy. Thereafter the infusion was adjusted to 0.5 μg/kg/h sufentanil and 0.02 mg/kg/h midazolam and maintained until the end of the operation. Dopamine (3 μg/kg/min) was used throughout the procedure for maintenance of adequate urine output. After termination of CPB, the infusion rate was adjusted according to the patient's circulatory state. Aprotinin (Trasylol®, Bayer, Leverkusen, Germany) was given according to the Hammersmith protocol [10].
A pulmonary artery catheter (7.5 F, Baxter, Irvine, CA, USA) via the right internal jugular vein through an introducer-sheath (8.5 F, Arrow, Reading, MA, USA) and two large-bore intravenous catheters were placed. Additionally, a combined fiberoptic-thermistor catheter 4 F was inserted via an introducer sheath into the femoral artery and advanced 40 cm up to the thoracic aorta for measurement of EVLW by the double indicator technique [11]. Care was taken that the position of the catheters did not change during the study.
For hemodynamic measurements, a commercial monitoring system was used (Solar 8000, Marquette Electronics, Inc.). Measurements of standard hemodynamic variables included heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP), mean pulmonary artery pressure (MPAP), pulmonary capillary wedge pressure (PCWP) and cardiac output. Cardiac output was measured in triplicate by thermodilution technique using boli of 10 ml cold sodium chloride (0.9%). Cardiac index (CI), systemic vascular resistance index (SVRI) and pulmonary vascular resistance index (PVRI) were calculated by the Marquette system.
EVLW was measured in triplicate. A bolus of 12 ml ice-cold indocyanine green (1.25 mg/ml ICG) was injected into the right atrium. For the assessment of the aortic dilution curves of the dye and the temperature and calculation of EVLW from these curves, a commercial device (Pulsion-COLD system, Pulsion, Munich, Germany) was used, which is described in detail elsewhere [11].
All measurements for cardiac and pulmonary function as well as EVLW of the patients were performed during continuous mandatory ventilation with an FiO2 of 1.0 and zero positive end-expiratory pressure.
2.1 Pre-CPB fluid management
After induction of anesthesia until the skin incision, 500 ml of HES 6% 200;0.5 was infused. Ringer's lactate (500 ml) was given after skin incision until the onset of CPB.
2.2 Cardiopulmonary bypass technique
The CPB equipment consisted of roller pumps (Stöckert, München, Germany), a disposable membrane oxygenator in combination with the cardiotomy reservoir and a tubing set (Dideco, Mirandola, Italy). The ECC circuit was primed according to the patients group (Table 1). For CPB, standard cannulation of the ascending aorta and the right atrium (two-stage venous cannula) was performed. Cardiopulmonary bypass was instituted at a flow rate of 2.4 l/min per m2 body surface area (BSA) upon systemic heparinization. The body temperature was reduced to 32°C by cooling on bypass. After cross-clamping the aorta, 1000–1500 ml of ice-cold crystalloid cardioplegic solution (Bretschneider=Custodiol®, Köhler Chemie, Alsbach-Hähnlein, Germany) were initially administered into the aortic root and supplemented as necessary.
Crystalloid as well as non-crystalloid fluid balances at the end of CPB were calculated. The crystalloid fluid balance equals the sum of crystalloid infusions, the crystalloid portion of the priming volume and the cardioplegia minus the urine output; the non-crystalloid fluid balance equals the HES-volume given minus the residual blood volume of the venous reservoir.
2.3 Post-operative care
The physicians responsible for post-operative care of the patients were blinded with respect to the study group. In the intensive care unit, the patients were sedated using 0.25 μg/kg/h sufentanil and 0.01 mg/kg/h midazolam and artificial ventilation was maintained with an FiO2 of 1.0 for 4 h. The sedation was then stopped, if the following criteria were fulfilled: the patient is hemodynamic stable using dopamine and/or dobutamine ≪10 μg/kg/min; thoracic drainage ≪50 ml/h; PCWP ≪20 mmHg; rectal temperature, ≫36.5°C. Extubation was performed if the patient was awake and the arterial pO2 ≫80 mmHg, pCO2 ≪50 mmHg, tidal volume ≫7 ml/kg using spontaneous breathing at Y-piece with 6 l/min O2.
Colloidal fluids (HES 6% 200;0.5) were given to maintain a CVP of about 10 mmHg, non-colloidal fluids and furosemid were given to achieve a negative crystalloid fluid balance of about 1000 ml until the first post-operative day.
2.4 Blood analyses
Arterial and mixed venous blood samples were obtained for gas analyses (Ciba Corning, Medfield, MA, USA) and measurement of the COP. Plasma COP was determined by a commercial membrane osmometer (Osmomat 050, Gonotec, Berlin, FRG) using a membrane with molecular mass cut-off at 20 kDa. Mixed venous oxygen saturation was measured using an oxymeter (Radiometer, Copenhagen, Denmark). Alveolo-arterial oxygen difference (AaDO2) and pulmonary shunt (Qs/QT) were calculated using standard formulae, as described previously [12].
2.5 Study protocol
Blood samples were obtained: (1) after induction of anesthesia before skin incision; (2) 15 min after the onset of CPB following cardioplegic cardiac arrest; (3) before weaning from CPB; (4) 2 h after CPB and (5) 4 h after CPB. Hemodynamic measurements were performed before skin incision and 2 h as well as 4 h after CPB, when the hemodynamic conditions were stable.
2.6 Statistics
Data are presented as mean±SD. The data were subjected to the Friedman two-way ANOVA. Comparison within groups were assessed with the Wilcoxon-matched-pairs signed-rank test and comparisons between groups with the Mann–Whitney U-test using the statistic software SPSS® 10.1 for Windows™. Significant differences were accepted for a tailed P-value of ≪0.05.
3 Results
Demographic data, pre-operative left ventricular ejection fraction (LVEF), ECC-time, cross-clamp time and the number of distal anastomoses were equal for patients in the two groups (Table 2 ).
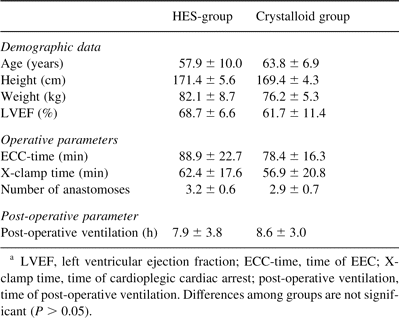
Demographic data and operative parameters of the patients (mean±SD)a
Following cardioplegic cardiac arrest, COP was decreased by 54% of the pre-operative value in the crystalloid group, but only by 20% in the HES-group. COP continuously increased after this in both groups and pre-bypass values were reached at 2 and 4 h following ECC, respectively (Fig. 1 and Table 3 ). At all time points during and after CPB, COP was significantly higher in the HES-group than in the crystalloid-group.
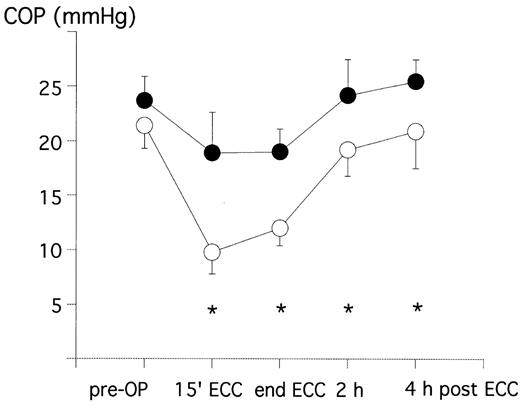
Plasma COP pre-operative (pre-OP), during ECC and in the intensive care unit in patients treated with crystalloid prime (crystalloid group=open circles) or colloid prime (HES-group=closed circles). *P < 0.05 between the two groups.

EVLW was increased by more than 20% in the crystalloid-group (from 5.4 ± 1.4 ml/kg BW to 6.5 ± 1.7 ml/kgBW) in the early post-operative period, whereas a trend to even reduced EVLW (from 5.4 ± 1.4 ml/kg BW to 6.5 ± 1.7 ml/kg BW) was present in the HES-group. The difference between groups was significant at 2 h following CPB (Fig. 2 and Table 4 ).
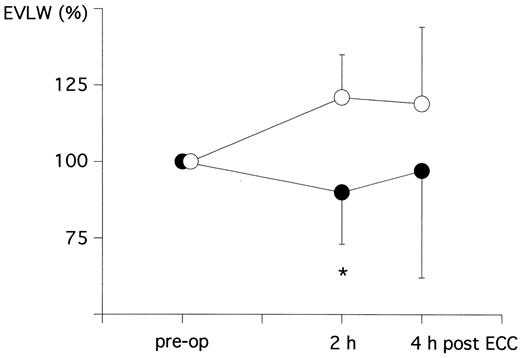
Change of EVLW as percent of the pre-operative level (pre-OP=100%) at 2 h as well as 4 h following ECC in patients treated with crystalloid prime (crystalloid group=open circles) or colloid prime (HES-group=closed circles). * P < 0.05 between the two groups.
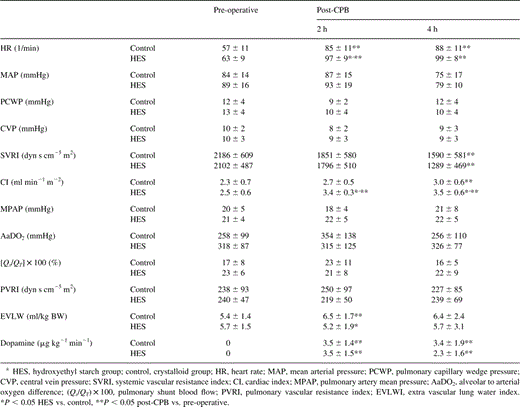
Data on cardiac and pulmonary hemodynamics and on pulmonary function pre-operative as well as 2 and 4 h following cardiopulmonary bypassa
The cardiac index was increased in both groups following CPB. However, this increase was significantly more pronounced, when HES was used (from 2.3±0.7 to 3.0±0.6 l/min/m2 and from 2.5±0.6 to 3.5±0.6 l/min/m2, respectively, at 4 h following CPB) (Fig. 3 and Table 4).
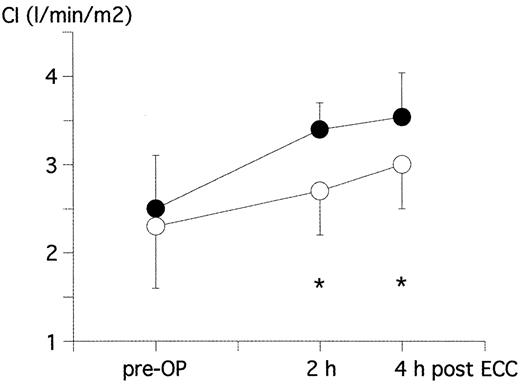
CI pre-OP and 2 h as well as 4 h following ECC in patients treated with crystalloid prime (crystalloid group=open circles) or colloid prime (HES-group=closed circles). *P < 0.05 between the two groups.
Intra-operative fluid balance, post-operative blood loss and post-operative gain of body weight of the patients are summarized in Table 5 . Priming volume, the amount of cardioplegia and post-operative blood loss was similar in the two groups. There was no bleeding complication leading to rethoracotomy. The residual blood volume of the venous reservoir was about doubled in the HES-group, when compared to the crystalloid group. The crystalloid fluid balance at the end of CPB was doubled in the crystalloid group, when compared to the HES-group. This resulted in a significantly higher net fluid balance in the crystalloid group than in the HES-group (Table 5). Accordingly, a post-operative gain in body weight was present in the crystalloid group, but not in the HES-group (Table 5).
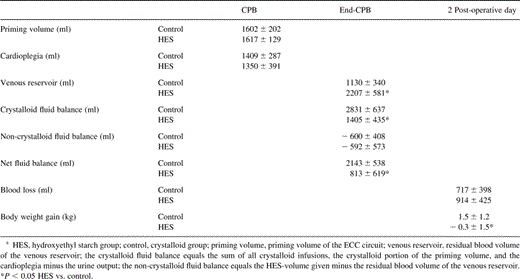
Data on intra-operative fluid balance, blood loss and patients gain of body weight following CPBa
Heart rate was increased following CPB in both groups. At 2 h post-CPB, the increase was significantly more pronounced in the HES-group. The SVRI was significantly decreased at 4 h after CPB in both groups without differences between the groups. PVRI, AaDO2, and pulmonary shunt blood flow (Qs/QT) did not change peri-operatively in either group (Table 4).
No differences between the two groups were seen for arterial and pulmonary artery pressures (PAP), PCWP and CVP (Table 4).
The post-operative administered dosage of dopamine was similar in the two groups (Table 4).
4 Discussion
Our data show that a hyperoncotic prime of the CPB circuit using HES 10% improves cardiac output and prevents accumulation of EVLW in the early post-pump period. A number of studies using various types of colloidal prime compositions (albumin, dextrans, gelatins, different HES preparations) have been undertaken to assess cardiopulmonary function and clinical outcome of the patients [7,13–18]. It still remains unclear, however, whether or not a colloidal priming of the ECC is superior to crystalloid solutions. Moreover, in addition to the ‘crystalloid vs. colloid priming’ controversy, there is an ongoing ‘colloid vs. colloid’ controversy, which was recently reviewed and discussed in detail by Boldt [6]. No beneficial effects with regard to clinical parameters and outcome of the patients could be demonstrated in the majority of the studies using colloidal prime compositions. One reason for that may be the fact that the COP of the priming solutions used was physiologic or less than that. Consequently, the initiation of the CPB and in addition the infusion of 1–2 l of crystalloid cardioplegic solution still lead to a considerable fall of the COP in the patients. Even in the present study, where a hyperoncotic CPB prime was used, the COP was reduced by about 20% 15 min after the the onset of CPB and after the application of 1000–1500 ml of crystalloid cardioplegia as compared to the pre-operative values.
In animal experiments, an isooncotic as compared to a crystalloidal prime of CPB attenuated an increase in water content of duodenum and skeletal muscle [19] and addition of hypertonic NaCl, hyperoncotic HES to an isotonic, hypooncotic prime of CPB prevented an increase in intracranial pressure and cerebellar water content [20].
An investigation using a hyperoncotic priming solution in a clinical study with patients undergoing CABG was performed by Jansen et al. [14]. To the best of our knowledge, this is the only study demonstrating an improved clinical performance score as well as a reduced post-operative hospital stay for patients treated with a colloidal CPB-prime. The clinical performance score included fluid balance, post-operative duration of intubation and the difference between the rectal temperature and skin temperature. Superior hemodynamic effects of the colloidal prime compared to the crystalloid prime, however, could not be demonstrated possibly due to the fact that inotropic support was guided by the cardiac index, which was not the case in our series. In the present study, the increase in cardiac index was significantly more pronounced, when a hyperoncotic CPB-prime was used (Fig. 3). A favourable effect on cardiac index could also be demonstrated by London et al. using pentastarch, but not by using albumin [17]. The reason for the positive effect on cardiac index in the HES-group is rather unclear. There is a slight, but insignificant trend to reduced systemic and pulmonary vascular resistance in the HES group. The filling pressures as well as the arterial and PAPs do not systematically differ among the groups.
Jansen et al. used 1650 ml of a priming solution with a COP of 32 mmHg [13]. In the present study, the CPB circuit was primed with 1617 ± 129 showing a COP of 48 mmHg, which is about twice the physiological value. Whereas Jansen et al. could demonstrate an increase of COP with the onset of CPB, our data show a 20% reduction of COP during the ECC. The difference between the data, however, can be readily explained. The time points of blood sampling are different in the two studies. Jansen et al. measured COP 5 min before and 5 min after the onset of CPB. It is likely that the blood sampling was performed before the cardioplegia was given, though it was not mentioned in the paper. In the present study, COP was measured before skin incision and 15 min after the onset of CPB. Between these time points, 500 ml of Ringer's lactate was given and 1000–1500 ml of cardioplegic solution was infused. Moreover, Jansen only used 800–1000 ml of cardioplegia instead of 1000–1500 ml used in our study.
During CPB, a significant difference of the COP between the crystalloid group and the colloidal group could be demonstrated in our study. In the HES-group, COP was reduced by 20%, while it fell by more than 50% in the crystalloid group. COP stayed significantly lower in the crystalloid group than in the HES-group but the difference between the two groups constantly decreased until 4 h post-CPB (Fig. 1 and Table 3). This phenomenon goes in line with the time course of the oncotic effect of HES, being maximal for 3–4 h after infusion and is then fading away.
In principle, we could prevent post-operative accumulation of EVLW in the early post-pump period. The pulmonary function, however, was surely not affected in the two groups because both the reduced EVLW-levels in the HES group as well as the elevated EVLW-levels in the crystalloid group still stayed within the normal range, which is 5–7 ml/kg BW. [11]. Therefore, it is quite plausible that no change in AaDO2 and pulmonary shunt fraction occurred in either group and it is also not surprising that the time of post-operative ventilation did not differ among the two groups (Table 2). This was also seen by Jansen et al. [14]. Low post-operative tissue water accumulation by minimizing the peri-operative crystalloid fluid balance and the fall of COP can be of benefit, however, in patients with pre-operative pulmonary and cardiac dysfunctions.
5 Summary and conclusions
Our data show that a hyperoncotic prime of the CPB circuit using HES 10% improves cardiac output and prevents accumulation of EVLW and weight gain in the early post-pump period in low-risk CABG patients. The reason for the improvement of the cardiac output after hyperoncotic CPB prime is unclear. Although a significant rise in EVLW occurred in the crystalloid group following CPB, the EVLW-levels did not exceed the physiological range. It is plausible, therefore, that pulmonary function was unaffected in either of the groups.
Further research has to be done to evaluate a possible beneficial clinical effect of post-pump improved cardiac output and low EVLW-levels for high-risk patients with impaired cardiac or pulmonary function or both and pre-operative elevated levels of EVLW.
Dr J. van der Linden (Stockholm, Sweden): I would like to ask, did you see any difference in clinical parameters like time of extubation of weight difference between the patients?
Dr Eising: The outcome of the patients was similar in the two groups because we examined relatively healthy patients. We saw a statistically significant difference in the weight gain after the procedure. The control group gained weight of about 1.8 kg at the second post-operative day, while we could totally prevent this weight gain in the hydroxyethyl starch group.
Dr van der Linden: Did you look at the amount of diuretics you needed?
Dr Eising: We only used furosemide in the post-operative course, and there was no difference between the two groups regarding the amount of diuretics.
Dr van der Linden: So you actually expect a bigger difference if you had had worse patients?
Dr Eising: We did this study more or less as a preliminary study. We now have to focus on patients with impaired organ function. We indeed expect more pronounced differences in the clinical outcome of those patients.
Dr A. Corno (Lausanne, Switzerland): It is well known that the amount of extravascular ling water content is dependent on several other variables during bypass other than osmotic pressure, for instance, the way you contact the cardiopulmonary bypass, temperature, flow, hematocrit and so forth, like the maintenance of low frequency ventilation or the urinary output or the utilization of ultrafiltration. Can we presume then in both groups you maintained stable all these variables during cardiopulmonary bypass, yes or not?
And the second question is, as a result of your observation, do you suggest HES priming for all patients or only for high risk patients?
Dr Eising: As this is a clinical investigation, you can never be sure that everything is a hundred percent systematic, but we tried to have a very consistent anesthesiologic regimen. All of the patients were treated the same way in terms of fluid input.
In response to your second question, we have not yet decided whether we should change our priming solution to a hyperosmotic prime. We first have to look at the older patients and the patients with impaired organ function.
References
- oxygen
- coronary artery bypass surgery
- cardiopulmonary bypass
- edema
- lung
- congestive heart failure
- anastomosis, surgical
- colloids
- demography
- extravascular lung water
- hetastarch
- indocyanine green
- osmotic pressure
- plasma
- pulmonary wedge pressure
- weight gain
- heart
- ice
- organ failure
- water balance
- respiratory shunt
- cardiac index
- systemic vascular resistance
- pulmonary artery pressure
- lactated ringer's solution
- crystalloid solutions
- pulmonary function
- mean arterial pressure
- pulmonary vascular resistance
- dilution technique




