-
PDF
- Split View
-
Views
-
Cite
Cite
John G. Byrne, Tomislav Mihaljevic, Wayne E. Lipson, Brian Smith, John A. Fox, Sary F. Aranki, Composite stentless valve with graft extension for combined replacement of the aortic valve, root and ascending aorta, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 252–256, https://doi.org/10.1016/S1010-7940(01)00799-0
Close - Share Icon Share
Abstract
Objective: The composite mechanical valve conduit has been most commonly used for patients who require combined aortic valve, root, and ascending aorta replacement, but is limited, especially in the elderly, because of the need for long-term anticoagulation. We report the first consecutive series of patients in whom a composite stentless valve with graft extension, which does not require long-term anticoagulation, was performed. Methods: Between April 1998 and July 2000, eight patients with severe aortic root and ascending aortic pathology underwent a combined aortic valve, root, and ascending aorta replacement with a Freestyle® stentless porcine valve with a Hemashield® graft extension. Mean age was 74 (range 56–82), three were males. Concomitant procedures included coronary artery bypass graft (CABG) alone (n = 2), mitral valve replacement with atrial septal defect repair (n = 1) and CABG with septal myomectomy (n = 1). Results: Operative mortality was zero. Median aortic cross-clamp and cardiopulmonary bypass times were 150 and 203 min, respectively. Two patients returned to the operating room for bleeding. Median blood transfusions and hospital length of stay were 4 units and 11 days, respectively. Conclusions: The composite stentless valve with graft extension is a reasonable alternative to a mechanical valve conduit for patients who require a combined aortic valve, root, and ascending aorta replacement, in whom anticoagulation is not desirable or contraindicated.
1 Introduction
The available choices for aortic valve replacement (AVR) include mechanical, porcine (stented and stentless), pericardial, homograft, and autograft valves. This assortment allows the surgeon to tailor the choice of AVR to the particular needs of each patient. In contrast, for patients with combined aortic valve and root pathology, in whom valve, root, and ascending aorta replacement is needed, replacement options are practically limited to mechanical valve conduits or homografts.
When long-term anticoagulation is not contraindicated, the composite mechanical valve conduit is probably the best choice. However, especially in the elderly, when long-term anticoagulation is not desirable or contraindicated, a ‘tissue valve conduit’ would be desirable. Currently, other than homografts with long aortic components, such a device is not commercially available in the United States. Those constructed in the operating room – consisting of a stented xenograft and a tube graft – require complete re-replacement of the aortic root in the event of late valve degeneration [1]. Previous isolated case reports have described the combined use of a Freestyle® (Medtronic, Minneapolis, MN, USA) stentless porcine valve with a Hemashield® (Boston Scientific/Meadox, Oakland, NJ, USA) tube graft extension [2,3], but a consecutive series of patients has yet to be reported. The stentless valve provides excellent hemodynamics as well as the possibility to re-replace just the porcine valve and not the entire conduit in the event of late valve degeneration [4]. In this report we document our experience with eight consecutive patients in whom this technique was used.
2 Materials and methods
Between April 1998 and July 2000, a composite aortic valve, root, and ascending aorta replacement was performed on 107 patients, 99 (93%) with St. Jude® (Minneapolis, MN, USA) valve conduit and eight (7%) with a Freestyle® stentless porcine valve with Hemashield® graft extension. None of the patients was excluded. Median age of the eight consecutive patients was 74 (range 56–82). Three were males. All patients had severe aortic insufficiency and aneurysmal disease. Ejection fraction ranged from 20 to 60%, median 55%. Table 1 summarizes patient characteristics.
2.1 Technique
The operation was typically performed through a full sternotomy, although a minimally invasive upper hemi-sternotomy approach was used in selected patients and if no concomitant procedures were required [5,6]. The distal ascending aorta or the proximal aortic arch was cannulated directly, as was the right atrium for cardiopulmonary bypass. Moderate systemic hypothermia (25–28°C) was used and, if circulatory arrest was anticipated, the patient was cooled to 15–20°C, depending on the expected duration of circulatory arrest. The presence of aortic regurgitation mandated immediate cross-clamping of the aorta and venting of the left ventricle following the onset of ventricular fibrillation. Myocardial protection was achieved with antegrade and retrograde cold blood cardioplegia. Concomitant procedures, such as distal coronary artery bypass graft (CABG) anastomoses or mitral valve surgery, were performed first.
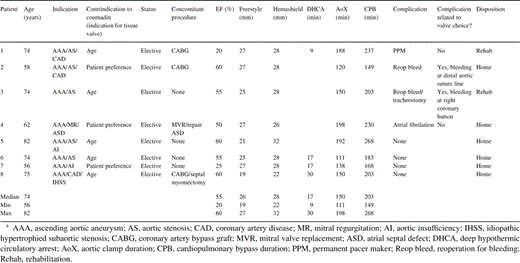
The ascending aorta was completely excised from just below the cross-clamp down to the level of, and including, the aortic sinuses. The right and left coronary buttons were isolated on a cuff of aortic wall. The aortic valve leaflets were excised. The Freestyle® valve sizers were used to select the appropriate valve. The proximal suture line was performed with interrupted mattress sutures of 4-0 Ethibond® (Ethicon, New Brunswick, NJ, USA) on an RB needle. The sutures were passed through the sewing ring of the valve (the horizontal green lines on the sewing ring indicate the level of cuspal attachments) and the valve seated and the sutures tied and cut. The left and right coronary buttons were anastomosed tension free to the appropriate position on the Freestyle®. A Hemashield®-tube graft was selected, typically 1–3 mm greater in diameter than the valve size, but primarily determined by the size of the distal aorta. For patients with aneurysmal extension beyond the cross-clamp, a period of circulatory arrest was required in order to excise the aneurysm and perform the distal anastomosis between the remnant of the ascending aorta or aortic arch and the Hemashield®-tube graft. Under these circumstances, the aortic cannula was then removed, along with the aneurysm, and later placed directly into the Hemashield®-tube graft. The distal aorta–tube graft anastomosis was performed in an end-to-end fashion with 4-0 Prolene® (Ethicon, New Brunswick, NJ, USA). During the period of circulatory arrest, the patient was maintained in the Trendelenburg position with continuous retrograde cerebral perfusion, to aid in preventing air embolism and to washout atherosclerotic debris. The circulation was restarted slowly expelling air from the aorta and the graft. The cross-clamp was then applied to the Hemashield®-tube graft. The proximal anastomosis between the Freestyle® and the tube graft was performed with running 4-0 Prolene® during which the patient was rewarmed (Fig. 1 ). Prior to tying the suture, the lungs were inflated, the venous return reduced, and de-airing performed. Before removing the cross-clamp, Tisseel® (Baxter Healthcare, Deerfield, IL, USA) fibrin glue was applied to all suture lines. After weaning from bypass, transesophageal echo was used to verify proper valve and cardiac function (Fig. 2 ).
(A) The arterial cannula has already been moved to the Hemashield®-tube graft, which has been anastomosed to the distal ascending aorta. The Freestyle® porcine valve is used to replace the aortic root. The right coronary button is anastomosed to the appropriate position on the Freestyle® valve. (B) The Freestyle® valve is anastomosed to the Hemashield®-tube graft with running 4-0 Prolene®. This is the only additional step required compared to using a mechanical valve conduit. (C) The completed composite replacement of the aortic valve, root, and ascending aorta is shown.
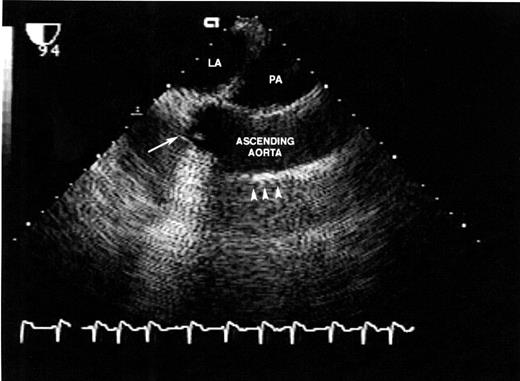
Post-operative transesophageal echocardiogram of final reconstruction: LA, left atrium; PA, pulmonary artery; long arrow, Freestyle® aortic valve; short arrows, Hemashield®-tube graft.
3 Results
Concomitant procedures included CABG alone (n = 2), mitral valve replacement with repair of an atrial septal defect (n = 1), and CABG with septal myomectomy (n = 1). There was no specific trend in valve or conduit size. Valve sizes ranged from 19 to 27, median 26, whereas conduit sizes ranged in size from 22 to 32, median 28. Median cross-clamp and cardiopulmonary bypass times were 150 and 203 min, respectively. Circulatory arrest was required in four patients, range 9–30 min, median 17 min (Table 1). Median post-operative blood transfusion requirement was 4 units per patient. Operative mortality was zero. Major complications occurred in three out of eight patients. One patient required a permanent pacemaker due to heart block, one required reoperation for bleeding and a tracheotomy for respiratory failure, and another required reoperation for bleeding. In one patient, bleeding was noted at the distal aortic tube graft anastomotic site and in the other patient bleeding occurred at the right coronary button. Median length of stay was 11 days and two patients required rehabilitation, while six were discharged home.
4 Discussion
A ‘tissue valve conduit’ that incorporates excellent hemodynamics and the possibility of valve re-replacement, should late degeneration occur, would be the ideal substitute for the aortic valve, root, and ascending aorta in patients in whom long-term anticoagulation is not desirable or contraindicated. Homograft aortic valves would provide an excellent choice but are limited by availability, especially in emergency situations, cost, and length of the aortic wall component. The Freestyle® valve is similar to aortic homografts in that it can be inserted in the subcoronary position, as a mini-root or as a full root [7]. Currently, the Toronto SPV® (St. Jude Medical, Minneapolis, MN, USA) is the other Food and Drug Administration- (FDA-) approved stentless valve but the SPV® valve can only be inserted in the subcoronary position. Subsequently, the majority of patients, particularly those in emergent situations such as type A dissections in elderly patients, end up receiving a mechanical valve conduit even when long-term anticoagulation is not desirable.
Composite stented xenograft valves with graft extension, although not commercially available in the United States, are limited by the need to replace the entire conduit in the event of late valve degeneration [1]. Thus, the ideal substitute for the aortic valve, root, and ascending aorta is not currently available.
Recently, Urbanski and Hacker [8] described the replacement of the aortic valve and ascending aorta with a stentless valve composite graft using a Toronto SPV® valve with graft extension. Their original technique was modified because of problems with bleeding [8,9]. They currently place the SPV® valve 3 mm above the end of the tube graft and use a 3-mm rim to sew the tube graft to the aortic annulus. The SPV® valve is then secured within the tube graft. Their technique is similar to ours in that a stentless valve is combined with a tube graft. However, because the valve is placed 3 mm above the level of the annulus, one must be very careful about the placement of the coronary buttons on the tube graft, because the buttons will either be very close to the valve or potentially kink due to the 3-mm displacement. In Urbanski and Hacker's report, they describe a 10% (2/20) incidence of the need for CABG because of technical complications. With the technique we describe, this should not be a problem because there is no artificial displacement of the valve above the annulus.
The rate of major complications in this small series was 38% (three out of eight patients – one patient had two complications). However, there were no deaths. Two of the major complications were related to the operational technique, both of which are reoperations for bleeding. The need for a permanent pace maker (PPM) for heart block and a tracheostomy for respiratory failure were unlikely to be related to the valve choice per se. A report by Kouchoukos et al. [10] on 172 consecutive aortic root replacements documented a cumulative major complication rate of approximately 25%: reoperation for bleeding 9%, need for IABP/VAD 4%, need for PPM 3%, need for tracheostomy 3%, and major neurological complications 6%. As documented in Table 1, all major complications in our series occurred early in our experience. As these are complex operations, it is no surprise that the rate of major complications may be significant, as previously documented by Kouchoukos.
Previous isolated case reports [2,3] have described techniques similar to the one reported here, but no consecutive series has been reported thus far. Our technique is analogous to placement of a mechanical valve conduit except that it involves one additional suture line (Freestyle®-tube graft anastomosis) which should take no more than a few minutes. Furthermore, the Freestyle® valve offers excellent hemodynamics [4]. If late valve degeneration necessitates valve re-replacement, then the previous Freestyle®-tube graft suture line can be taken down and a valve replacement with a stented valve can be carried out. Calcification of the porcine aortic wall may occur, as with homografts. However, Sundt et al. [11] recently documented the feasibility of valve re-replacement after homografts and the same principle may apply to the Freestyle® valve.
5 Conclusions
When replacement of the aortic valve, root, and ascending aorta is required in a patient in whom long-term anticoagulation should be avoided, then a composite stentless valve with graft extension is a reasonable alternative. It provides excellent hemodynamics, involves just one additional suture line compared to a mechanical conduit, and provides the possibility for valve re-replacement, without full conduit change, should that become necessary.
References
- anticoagulation
- aorta
- aortic valve
- coronary artery bypass surgery
- cardiopulmonary bypass
- hemorrhage
- conduit implant
- ascending aorta
- mitral valve replacement surgery
- blood transfusion
- length of stay
- operating room
- suidae
- tissue transplants
- pathology
- anticoagulation, chronic
- uterine myomectomy
- older adult
- supraaortic valve area
- closure of defect of interatrial septum
- surgical mortality
- replacement of ascending aorta




