-
PDF
- Split View
-
Views
-
Cite
Cite
Christos Alexiou, Qiang Chen, Stephen M. Langley, Anthony P. Salmon, Barry R. Keeton, Marcus P. Haw, James L. Monro, Is there still a place for open surgical valvotomy in the management of aortic stenosis in children? The view from Southampton, European Journal of Cardio-Thoracic Surgery, Volume 20, Issue 2, August 2001, Pages 239–246, https://doi.org/10.1016/S1010-7940(01)00813-2
Close - Share Icon Share
Abstract
Objective: The most appropriate management of aortic stenosis (AS) in children remains controversial. The purpose of this study was to determine the outcome following open valvotomy for AS in children. Methods: Ninety-seven consecutive, unselected, children (mean age 3.2±3.6 years, 1 day–15 years) underwent an open valvotomy for critical (n = 36) or severe (n = 61) AS between 1979 and 2000 in Southampton. Twenty-six were neonates (1–31 days), 27 were infants (1–12 months) and 44 were older children (1–15 years). Mean follow-up was 10±5.4 years, 1 month–21.9 years. Results: Two neonates died early giving an overall operative mortality of 2.1% (7.7% for the neonates and 0% for infants and older children). The mean aortic gradient was reduced from 76 to 24.5 mmHg (P < 0.0001). Residual or recurrent AS occurred in 17 patients and severe aortic regurgitation in eight patients. Kaplan–Meier 10-year freedom from residual or recurrent AS was 83.1±4.7% and from severe aortic regurgitation was 95.3±2.7%. Twenty-five patients required an aortic re-operation or re-intervention, 18 of whom had an aortic valve replacement (AVR) (mean valve size 21.8±0.9 mm, range 21–25 mm). Ten-year freedom from any aortic re-operation or re-intervention was 78.4±5.2% and from AVR was 85.1±4.6%. There were ten late deaths. Overall 10-year survival, including hospital mortality, was 90.2±3.1% (69.7±9.7% for the neonates, 92±5.4% for the infants and 100% for older children, (P < 0.0001). Ten-year survival for children with isolated AS (n = 69) was 100% and for those with associated cardiovascular problems (n = 28) was 67.3±8.9% (P < 0.0001). All survivors are in New York Heart Association functional class I. Conclusions: Open valvotomy remains the gold standard in the management of AS in neonates, infants and older children. It is associated with low operative mortality and provides lengthy freedom from recurrent AS and regurgitation. Re-operations are common but if AVR is required, implantation of an adult-sized prosthesis is usually possible. There is a late death-hazard for those with severe associated lesions, but the survival prospects for the patients with isolated AS are excellent.
1 Introduction
Open surgical valvotomy has been successful in relieving the aortic obstruction but has traditionally carried a high operative risk, particularly in the neonates and young infants [1,2]. Percutaneous balloon valvotomy has been proposed as a less invasive option [3] but it has been initially linked with significant procedure-related morbidity and mortality [4]. More recent reports suggest that the results of open and percutaneous valvotomy are improving and they are, currently, both considered as safe and effective techniques [5–14].
Balloon valvotomy has over the last decade gained in popularity and is used in several centres as the primary treatment of aortic stenosis (AS) in children. In our unit, open surgical valvotomy under cardiopulmonary bypass (CPB) and cardioplegic myocardial arrest has since 1979 been the standard approach for the management of this condition.
We have previously described a group of 18 neonates having an open valvotomy for isolated AS in this unit [15]. The purpose of this study was to analyse the outcome achieved with this therapeutic strategy in an unselected series of neonates, infants and older children and to provide a set of data against which current results of balloon valvotomy can be evaluated.
2 Patients and methods
Between January 1979 and August 2000, 97 consecutive, unselected, children (68 males and 29 females) underwent an open surgical valvotomy for AS in Southampton. Their mean age was 3.2±3.6 years (range 1 day–15 years). Twenty-six of these children were neonates (mean age 8.3±6 days, range 1–31 days), 27 were infants (mean age 4.1±2.7 months, range 1–12 months) and 44 were older children (mean age 6.8±4.1 years, range 1–15 years). Since the presenting features and the outcome following aortic valvotomy at different ages are likely to differ, the age groups mentioned above (neonates, infants and older children) will be considered separately.
Until 1995, neonates with small-sized hearts and critical AS were, in this unit, treated with an open valvotomy in the hope that this would allow the heart to grow sufficiently to support the circulation. The management of this condition has since then evolved and infants having echocardiographical evidence of hypolastic left heart syndrome (aortic valve annulus <5 mm, mitral valve annulus <9 mm and left ventricular inflow diameter <21 mm) [16] are now managed with a Norwood type I palliative procedure.
No patient was excluded from this study on the grounds of the severity of his condition or the presence of associated lesions.
2.1 Definitions
Critical AS is characterised by a low cardiac output state accompanied by signs and symptoms of congestive cardiac failure. In this situation, urgent intervention to relieve the aortic valvular obstruction is mandatory.
Endocardial fibroelastosis (EFE) is focal or diffuse cartilage-like fibroelastic thickening of the mural endocardium restricting the diastolic ventricular compliance and often obliterating part of the apical ventricular cavity. In this series, diagnosis in the living patient was made with echocardiography showing abnormal endocardial brightness. Its presence in those who died was confirmed with histopathological examination of endocardial specimens taken at post-mortem.
Operative mortality includes death occurring within 30 days after the operation or during the same hospital admission. Deaths taking place more than 30 days post-operatively following the patient discharge from the hospital are termed late deaths.
Patients having aortic coarctation, atrial septal defect (ASD) or patent ductus arteriosus (PDA) as the only co-existing defect were, for the purpose of survival analysis, considered as having ‘isolated’ AS as previously suggested [5].
2.2 Statistics
Continuous data are presented as means (±standard deviation) and categorical variables as percentages. Means were compared with paired t-test and proportions with chi-square test. The prediction of freedom from late complications, re-operation and death (±standard error from the mean) was estimated with the Kaplan–Meier product limit method and the resulting curves compared with the log–rank test. The level of statistical significance was set at a P value of less than 0.05. Analysis was done with the statistical package SPSS PC (version 8.0) (SPSS Inc., 444 N. Michigan Avenue, Chicago, IL 60611, USA).
2.3 Pre-operative clinical features
Routine pre-operative evaluation included two-dimensional echocardiography with Doppler studies. Thirty-six patients (all 26 neonates, eight infants and two older children) had critical AS. Severe AS was the indication for an open valvotomy in the remaining 61 patients. The operative procedures were non-elective (urgent or emergency) in 53 cases (26, 13 and 14). The mean aortic valve gradient was 74.7±15.8 mmHg (70.7±17.3, 84.8±13.6 and 70.8±14.2) ranging from 30 to 125 mmHg (30–120, 50–125 and 40–100 mmHg). Thirty-nine patients (21, 14 and four) had impaired left ventricular contractility (ejection fraction <50%) and nine patients (six, three and zero) had left ventricular dilatation on pre-operative echocardiography.
According to the criteria suggested by Turley et al. [5], 69 patients (18, 15 and 36; P = 0.2) had ‘isolated’ AS. If all abnormalities were taken into account (i.e. including aortic coarctation, ASD, patent foramen ovale (PFO) and PDA), then 46 patients (19, 14 and 13; P = 0.001 had 85 coexisting defects (Table 1 ).
Eleven patients (one, four and six) had undergone 16 previous cardiovascular operations. These were repair of aortic coarctation (one, two and two), closure of ventricular septal defect (VSD) (zero, one and two), repair of interrupted aortic arch (zero, zero and two), ligation of PDA (zero, two and two) homograft aortic valve replacement (AVR) (zero, zero and one) and pulmonary artery banding (one, zero and zero).
2.4 The operation, concomitant procedures and valvular pathology
The operations were performed through a median sternotomy under CPB, established with an ascending aortic and a single right atrial cannula [17]. Myocardial protection was provided with cold crystalloid or blood cardioplegia and topical hypothermia. After transverse aortotomy, the aortic valve was inspected and a careful commissurotomy down to the aortic annulus was performed. Obstructive myxomatous and fibrous nodules on the aortic valvular surfaces and the free edges were meticulously excised.
Nineteen patients (11, five and three) underwent 24 concomitant procedures. These were, repair of aortic coarctation (three, two and one), repair of interrupted aortic arch (one, zero and one), resection of subaortic stenosis (one, one and one), closure of ventricular septal defect (two, zero and one), pulmonary valvotomy (zero, one and zero) and ligation of PDA (seven, two and zero). Seventy-two patients (12, 22 and 38) had a bicuspid aortic valve, 24 (ten, nine and five) had a tricuspid and one older child had a unicuspid valve. Fibrous nodularities and excrescences on the aortic valvular surfaces or free edges of the leaflets were present in 29 cases (ten, nine and ten).
2.5 Follow-up
Patients were seen in the outpatient clinics at regular intervals by the paediatric cardiologists. Two-dimensional echocardiography and Doppler studies were routinely performed before discharge from the hospital and during each outpatient visit. Information was obtained from the medical records, the patient's general practitioner and families. Clinical information and echocardiographical data were available for all patients within 6 months from the closing date of this study (30/08/00). The mean follow-up was 10±5.4 years, range 1 month–21.9 years). As no neonate had an open valvotomy in our unit between 1979 and 1984, the length of follow-up in neonates (mean 5.9±5.1 years, 1 month–15 years), was shorter than in infants (mean 12.5±4.8 years, range 1.3–21.9 years) and older children (mean 10.9±5.2 years, range 1.9–21.5 years).
3 Results
3.1 Operative mortality and morbidity
Two patients died early and were both neonates. Overall operative mortality was 2.1% (7.7% for neonates, 0% for infants and 0% for older children). A 1-day-old neonate born with critical AS, hypolastic left ventricle and aortic arch, mitral stenosis and EFE was transferred from another hospital being hypotensive and anuric. He had peritoneal dialysis but he deteriorated and required valvotomy as an emergency. He died in the intensive care unit from cardiac failure. A 9-day-old neonate with critical AS, EFE, hypoplastic, non-compliant, left ventricle and hypoplastic aortic arch died 11 days after an emergency valvotomy due to low cardiac output, coagulase negative Staphylococcal septicaemia and multiple organ failure. Post-mortem examination confirmed on both occasions the echocardiographical and intra-operative findings.
Fourteen patients (11, two and one) experienced significant non-fatal early post-operative complications. In neonatal group, eight required inotropic support for more than 48 h after their operation and six were ventilated for at least 4 days. Two infants needed prolonged inotropic support and an older child had sternal re-opening for mediastinal bleeding. The remaining 81 patients (13, 25 and 43) (P < 0.0001) had an uneventful post-operative recovery.
3.2 Early functional outcome
Echocardiographical examination before the discharge from the hospital demonstrated a good left ventricular function in 82 patients (16, 23 and 43), slightly impaired but improved compared to the pre-operative status in 12 patients (seven, four and one) and persistently poor myocardial contractility in one neonate. The mean gradient across the aortic valve was reduced to 24.5±8.1 mmHg (range 0–47 mmHg) (28.9±8, 25.8±7.8 and 20.5±6.1). The reduction in the aortic valve gradient was highly significant for all the three age groups (P < 0.0001). Mild aortic regurgitation was present in fifteen (six, four and five) and moderate aortic regurgitation in four patients (two, one and one).
3.3 Recurrent aortic stenosis and aortic regurgitation
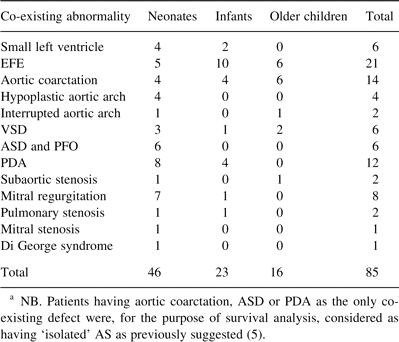
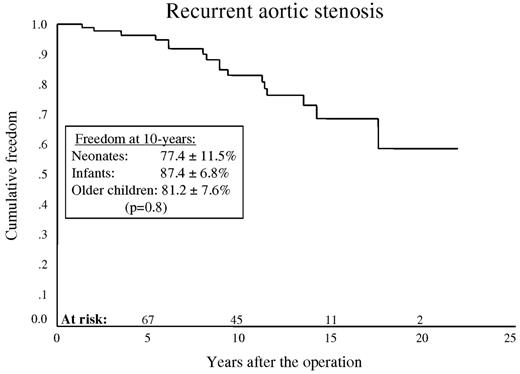
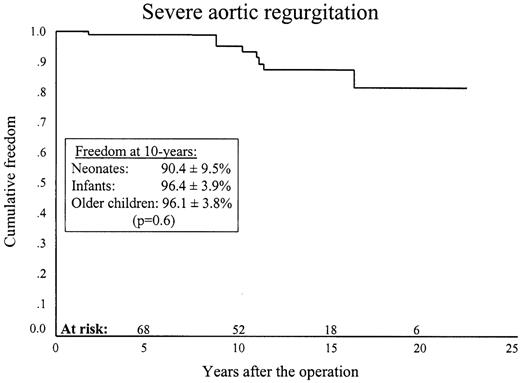
Recurrent or residual AS occurred in 17 patients (three, seven and seven) and severe aortic regurgitation in eight (three, four and one). Kaplan–Meier, 10-year freedom from recurrent stenosis was 83.1±4.7% (77.4±11.5, 87.4±6.8 and 81.2±7.6%) (P = 0.08) (Fig. 1 ) and from severe aortic regurgitation this was 95.3±2.7% (90.4±9.5%, 96.4±3.9% and 96.1±3.8%) (P = 0.06) (Fig. 2 ).
3.4 Re-operations or re-interventions
Twenty-five patients (six, 11 and eight) required 26 aortic re-operations or interventions with no operative mortality. These were AVR (three, eight and seven), repeat open aortic valvotomy (zero, one and one), repeat open aortic valvotomy and resection of subaortic stenosis (one, one and zero), aortic valve repair (two, zero and zero), aortic and mitral valve repair with mitral annuloplasty (one, zero and zero) and balloon valvotomy (zero, one and zero).
Ten-year freedom from any type of re-operation or re-intervention was 78.4±5.2% (66.3±14.2%, 83.5±7.5% and 77.4±8.2%) (P = 0.08) (Fig. 3 ).
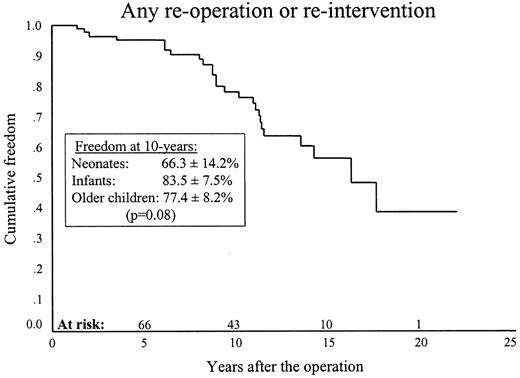
Ten-year freedom from re-operation or re-intervention was 78.4±5.2%.
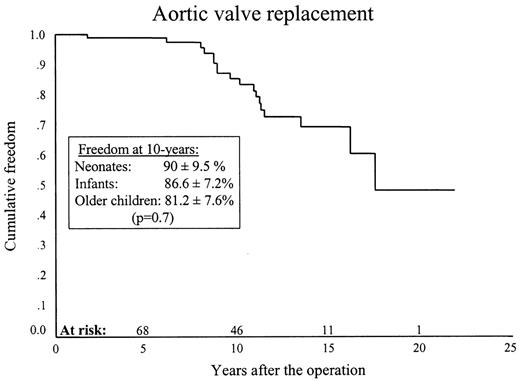
Eighteen patients (three, eight and seven) had an AVR. One patient who had an emergency aortic valvotomy at 6 weeks of life developed severe aortic regurgitation and underwent valve replacement with a pulmonary autograft at 2 years of age. The remaining 17 patients underwent AVR with a mechanical prosthesis. The mean size of the mechanical valves in the 16 patients who had a valve replacement in our unit was 21.8±0.9 mm (range 21–25 mm). The valve size in one patient who had an AVR in another unit is not known. Ten-year freedom from AVR was 85.1±4.6% (90±9.5%, 86.6±7.2% and 81.2±7.6%) (P = 0.7) (Fig. 4 ).
3.5 Late survival and functional status
There have been ten late deaths (six, four and zero). All deaths, but one, affected patients with co-existing cardiovascular abnormalities. The only death in a patient with isolated AS occurred in a 14-year-old boy who had a valvotomy as a neonate for critical AS in our unit and an AVR at ten years of age elsewhere. He died 4 years after his AVR from septic cerebral infarcts complicating Staphylococcal native mitral and prosthetic aortic valve endocarditis. Causes of late death and co-existing abnormalities are shown in Table 2 .

Overall 10-year survival, including hospital mortality, was 90.2±3.1% (69.7±9.7%, 92±5.4 and 100%) (P < 0.0001) (Figs. 5 and 6 ). Ten-year survival for the patients with isolated AS was 100% and for those having AS complicated by additional cardiovascular disorders, it was 67.3±8.9% (P < 0.0001) (Fig. 5). Ten-year survival for isolated vs. complicated AS for neonates, infants and older children was 100 vs. 0% (P < 0.0001) 100 vs. 83.3±10.7% (P = 0.4) and 100 vs. 100% (P < 1.0) respectively.
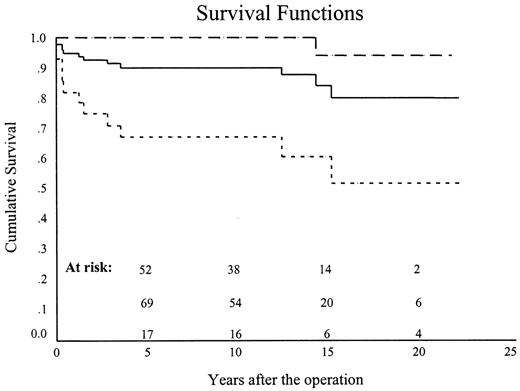
Overall ten-year survival (continuous line), including operative mortality, was 90.2±3.1%. Ten-year survival for the patients with ‘isolated’ AS (interrupted line) was 100% and for those having severe additional cardiovascular disorders (dotted line) it was 67.3±8.9% (P<0.0001).
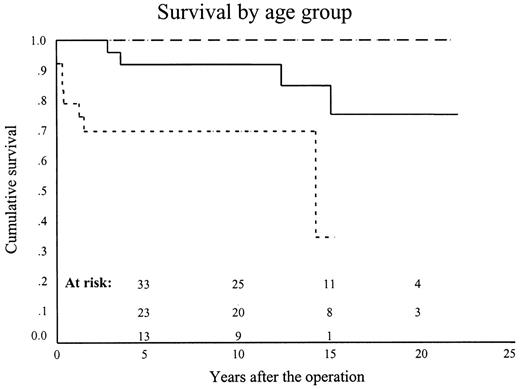
Ten-year survival for the neonates (dotted line), infants (continuous line) and older children (interrupted line) was 69.7±9.7%, 92±5.4% and 100% (P < 0.0001).
At the latest echocardiography the mean aortic valve gradient was 29.3±11.4 mmHg (30.3±10.3, 24.4±9.7 and 31±11.6 mmHg), range 0–62 mmHg with 22 patients (six, six and ten) having mild and 14 patients (five, three and six) having moderate aortic regurgitation. All the survivors are in the New York Heart Association functional class I.
4 Discussion
We believe that the merits of the available options for the management of AS in children should be evaluated on the basis of their ability to relieve the aortic obstruction and provide lasting freedom from residual or recurrent stenosis. It is important that this should be accomplished without causing severe aortic regurgitation, with the least possible procedure-related morbidity and mortality.
Use of more than one approach in other institutions may introduce patient selection or referral bias undermining the validity of attempted comparisons between techniques [8]. The value of this series lies in the unselected nature of the patients involved, since it includes all children requiring interventional treatment for AS in our unit.
4.1 Operative risk
The overall operative mortality of 2.1% in this series was low. Not surprisingly, both early deaths occurred in desperately sick neonates with small-sized hearts and EFE, even though adequate relief of the aortic obstruction has been achieved. The adverse prognostic nature of small-sized left ventricles, EFE and other co-existing defects has been well reported [16,18]. As discussed previously, from 1995, we have changed our policy and perform a Norwood-type procedure in the neonates and young infants with evidence of hypoplastic left heart syndrome on pre-operative echocardiography [16] and there has been no operative mortality since.
Our early mortality of 7.7% in neonates compares favourably with surgical mortality rates of over 50% quoted in the past [1,2]. Combined with early death rates of 9–18%, recently reported from other centres, our experience indicates that surgical valvotomy in this difficult group of patients is becoming safer [5–7,11].
The lack of operative mortality, in this series, among the infants over 1 month and older children was gratifying and compares favourably with mortality rates of 0–5% quoted for patients of similar age undergoing surgical valvotomy [1,19,20].
4.2 Surgical technique, re-operations and management of mitral regurgitation
Accepting that future valve replacement is almost certain, the goal of any type of treatment is to delay an AVR until the child grows sufficiently to accommodate a bigger size valve. Minor degrees of residual AS are usually well tolerated and may be amenable to repair but if severe aortic regurgitation occurs, AVR often becomes inevitable. Care should, therefore be taken, in performing the commissurotomy so as to avoid regurgitation. We believe also that removal of the fibrous excrescences and nodularities from the under-surface and the free edges of the aortic leaflets increases the effective aortic orifice area and consider it to be an important part of the operation [7]. This in itself can limit the need for an extended commissurotomy.
Our experience has, in this respect, been rewarding. There has been no case of severe aortic regurgitation or re-stenosis early post-operatively. With the exception of one patient who had an AVR with a pulmonary autograft 2 years after his valvotomy, 16 patients had a mechanical AVR more than 6 years later receiving at least a 21-mm size valve. By inserting an adult-sized prosthesis, we hope that many of these children might have had their last aortic operation. It is also encouraging that there has been no operative mortality associated with the AVR.
Age at initial valvotomy did not influence the time-interval until valve replacement. The prediction of ten-year freedom from any re-operation of 78% and from AVR of 85% is similar to figures quoted following open valvotomy at various age groups previously [5–7,19,20].
Others advocate the use of a pulmonary autograft for AVR in children, mainly, for its good haemodynamic characteristics and the benefits gained by avoiding the anticoagulants and prosthesis-related complications, and they report good early and mid-term results [21]. The Ross procedure, however, has the problems of involving a previously healthy right outflow tract and pre-disposing to further re-operations. In a recent long-term follow-up study, we have found that mechanical prostheses performed well in the aortic position, were associated with a low incidence of valve or anticoagulation-related events and provided good survival in children [22]. Modern low profile mechanical bileaflet valves are the valve substitute of our choice for paediatric AVR.
Mitral regurgitation may present a considerable additional haemodynamic problem particularly in the setting of critical AS. Before surgery we focus in treating the congestive heart failure and providing respiratory support as required. Subsequent relief of the AS would normally lead to a gradual reduction of the mitral regurgitation. Seven neonates and one infant with critical AS had also severe mitral regurgitation in this series (Table 1). None of these patients required concomitant mitral valve surgery but one did undergo late aortic valve repair, mitral valve repair and annuloplasty for recurrent AS and mitral regurgitation.
4.3 Late survival
The late mortality in neonates and infants, in this series, was relatively high but all, but one, deaths occurred in children with severe co-existing cardiovascular defects (Table 2), even though they did not have evidence of aortic valve dysfunction. This shows that the provision of intensive peri-operative care can avoid early mortality but there exists a late death-hazard for neonates and infants with hypoplastic hearts and other severe associated lesions. Infants and neonates with ‘isolated’ AS fared well with the only death among them being due to endocarditis following a mechanical AVR elsewhere. This reminds us of the well-known risk of infection of any type of prosthetic heart valve (mechanical, heterograft or homograft) but it is notable that none of the 56 children undergoing an AVR with mechanical prosthesis, between 1973 and 1999 in our unit, experienced such a complication [22].
The presence of associated conditions did not influence the early and late outcome among children older than 1 year at the time of the initial valvotomy and all of them remain alive and well. The ten-year survival for neonates, infants and older children of 70, 84 and 100% compares favourably with survival figures quoted for patients undergoing open surgical valvotomy in previous studies [5–7,19,20]. The ten-year survival of 100% for isolated AS for all three age groups is encouraging (Fig. 5).
4.4 Percutaneous balloon valvotomy
Percutaneous balloon valvotomy has been, initially, associated with a novel range of complications including transection of the femoral and iliac artery, perforation of the aorta, avulsion of the aortic valve leaflets, massive aortic regurgitation, perforation of the left ventricle, pericardial tamponade, perforation of the mitral valve leaflets, ventricular tachy-arythmias, stroke etc. [3,4,23] and early mortality rates in excess of 50% [4]. With increasing experience the incidence of these problems had been reduced but by no means eliminated, with the vascular complications, sometimes fatal, being still more pronounced in neonates [8,12,23].
As with surgical valvotomy, the operative risk is higher among the younger patients. In the neonatal age group Borghi et al. [12], Gatzoulis et al. [8] and Zeevi et al. [24] have reported early mortality rates of 20–25% following balloon valvotomy through a transfemoral approach. More recently, Robinson et al. [13] described 92 infants, 1–191 days of age, undergoing balloon valvotomy through a carotid cut-down with 13 early deaths. Operative mortality among the duct dependent infants was 38% and it was 5% for those not dependent on the arterial duct. Of note, several deaths in these series were due to procedure, examples being laceration of iliac vessels, avulsion of aortic cusps with gross aortic regurgitation and ventricular fibrillation from intracardiac catheter manipulation. McCrindle [23] has reported on 606 children who had a balloon valvotomy with a procedure-related mortality of 1.9%. This seemingly low but certainly not negligible mortality demonstrates that the ‘less invasive’ approach is not necessarily the safest. The neonatal operative mortality in our unselected series compares very favourably with that recently quoted for balloon valvotomy [8,12,13,24] and we did not have procedure-related deaths.
Balloon valvotomy effectively reduces the transvalvar gradient but has a disturbing rate of severe early aortic regurgitation. In the series of Robinson et al. [13], five patients had severe and ten had moderate early aortic regurgitation. Four of the patients with severe regurgitation died early and four of the patients with moderate regurgitation went on to develop severe regurgitation soon after their discharge from the hospital. Thirteen of the 60 children in the report of Hawkins et al. [25] developed severe regurgitation, all due to avulsion of the aortic cusps, requiring an aortic re-operation within 44 months. Two of the 16 patients in the series of Zeevi et al. [24] developed severe early regurgitation from which they died. Despite its problems balloon valvotomy has in several units superseded open valvotomy as the primary procedure for the management of paediatric AS.
We, in agreement with others, find that open commissurotomy under hypothermic CPB and cardioplegic myocardial arrest provides certain distinct physiological and anatomical advantages [5–7]. In the children with compromised haemodynamics, CPB allows for the myocardium to recover and for the deranged metabolic parameters to be corrected. It enables direct inspection of the aortic valve and facilitates the performance of an appropriate commissurotomy and thorough valve debridement. This is not achievable with any of the ‘blind’ methods of aortic dilatation techniques currently in use. The careful division of fused cusps over even as little as 1 or 2 mm can greatly increase the cross-sectional area of the valve and, thus, allow a marked increase in the cardiac output [15,17].
We feel that our surgical technique accounts for the lack of early, clinically significant, aortic regurgitation in this series. Severe aortic regurgitation can be very detrimental – it pre-disposes to an early re-operation and, as the experience with balloon valvotomy shows, may be the cause of unexpected early death [13,24].
5 Conclusions
In our view, open surgical valvotomy under CPB and cardioplegic myocardial arrest remains the gold standard in the treatment of AS in neonates, infants and older children. It can be accomplished with low operative mortality even in the critically ill neonates, providing lengthy freedom from aortic residual or recurrent stenosis and regurgitation.
Re-interventions are common but if an AVR is needed an adult sized prosthesis can usually be implanted. Children with severe associated cardiovascular problems fare less well but the survival prospects for those having isolated AS are excellent.
The authors wish to acknowledge the clinical contribution of Dr James Gnanapragasam, Mr Daryl F. Shore, Mr Robert K. Lamb, Mr Victor T. Tsang and Sir Keith Ross in the management of these children.
Dr U. Urban (St. Augustin, Germany): We are grateful for getting standards, probably gold standards from the Southamptom group to compare cardiological interventions in the aortic root. If I compare, you had a mortality of lower than 10% in the critical aortic stenosis group of the neonates, which matches very well with what cardiologists have today, and I think you had a far less degree of aortic in competence. Is that so?
Mr Alexiou: Yes, the neonatal operative mortality in this series compares well with that reported by the cardiologists. Also, early aortic regurgitation is a considerable problem after balloon valvotomy and it is important that none of our patients have developed early regurgitation.
Dr T. Tlaskal (Prague, Czech Republic): I have been rather surprised with your extraordinary results, especially in the subset of patients operated during neonatal period and infancy. Your very low mortality rate in neonates is remarkable. I would like to ask you three questions. Did you really perform surgical valvotomy in all patients with valvar aortic stenosis seen in your hospital or were some of them treated by balloon valvuloplasty? Would you kindly comment on your surgical approach, especially in neonates with dysplastic aortic valve? Do you have some special tricks? I must say that I am very surprised by the low rate of severe aortic regurgitation during the long-term follow-up in your series.
Mr. Alexiou: Open surgical valvotomy was the only therapeutic modality employed for the primary treatment of critical or severe aortic stenosis in neonates, infants and children over the study period in our institution.
Our interventional paediatric cardiologists appreciate that our surgical operative mortality is very low indeed, particularly in the neonates, and although they perform large numbers of intra-vascular and intra-cardiac procedures for other conditions, they do favour open surgical valvotomy for the treatment of aortic stenosis.
Very dysplastic valves such as unicuspid valves are difficult to deal with but there were no unicuspid valves in our series. Mr Monro has described a technique for the management of bicuspid valves (Ann Thorac Surg 1997;63:465–9). This involves suturing the base of a triangle piece of bovine pericardium with a vertical fold into the free edges of the incised raphe so as to render the valve tricuspid and trisinusoidal, recreating the deficient interleaflet triangle and preventing cusp prolapse.




