-
PDF
- Split View
-
Views
-
Cite
Cite
Robert A. Cesnjevar, Richard Feyrer, Friedrich Walther, Faidi O. Mahmoud, Yvonne Lindemann, Juergen von der Emde, High-risk mitral valve replacement in severe pulmonary hypertension—30 years experience, European Journal of Cardio-Thoracic Surgery, Volume 13, Issue 4, April 1998, Pages 344–352, https://doi.org/10.1016/S1010-7940(98)00042-6
Close - Share Icon Share
Abstract
Objective: In the past 30 years, 2316 patients underwent mitral valve replacement (MVR) at our institution; 382 of them had severe pulmonary hypertension (pulmonary artery pressure (PAP)≫50 mmHg; pulmonary vascular resistance (PVR), 690±46 dyn/s per m2). We reviewed our early and late results in this high-risk subgroup. Methods: We used 336 mechanical and 46 biological devices for MVR. The follow-up was 95%, with an observation period of 3208 patient-years and a mean of 8.4±0.2 years per patient. The overall early mortality rate was 10.5% (n=40) and stayed at about the same level over the years, although patients characteristics have changed to much older patients and more reoperations. To clarify this fact we divided our data in results according to the decades in which the operations were carried out. The clinical preoperative status and results were as follows (* P≪0.05; ** P≪0.01 compared with previous decade). In the decades between 1963 and 1973 (I), 1974 and 1983 (II) and 1984 and 1993 (III) we operated on n=95 (I), n=185 (II), and n=102 (III) patients with a mean age of 43±1 (I), 50±1** (II), and 58±1** (III) years. The incidence of reoperations among these patients was 3.2 (I), 4.9 (II), and 22.6%** (III). The early mortalities were 13.7 (I), 8.6* (II) and 10.8% (III); late mortalities lowered from 5.77 (I), over 4.95 (II), and up to 3.39%** (III) patients/year. The mean functional status according to New York Heart Association (NYHA) class improved from preoperatively 3.0±0.1 (I), 3.2±0.1 (II) and 3.3±0.1 (III) to 2.4±0.2 (I), 2.4±0.1 (II) and 2.3±0.1 (III) postoperatively. Results: Compared with routine elective MVR with a mortality rate of 3.6% (P≪0.01), early mortality is high. But once the patient survives the perioperative course, late results show no difference compared with patients without pulmonary hypertension. The functional results as well are not significantly different. In spite of on average 15 years older multimorbid patients with therefore higher complication rates, early results improved slightly, which could be explained by better operative techniques, perioperative treatment and nursing (online monitoring with immediate therapeutic substitution). Surprisingly the increased number of reoperations had no negative impact on patients’ outcomes. Conclusion: According to our results, we recommend MVR in severe pulmonary hypertension even in the elderly, with a high but acceptable risk and good long-term results.
Introduction
The first successful prosthetic replacement of an irreversibly damaged mitral valve was reported by Starr more than 35 years ago [1]. This historical surgical procedure marked the beginning of mitral valve replacement (MVR), and was followed by a large number of investigations and inventions. Ever since the beginnings of mitral surgery, severe pulmonary hypertension has been an important risk factor for patient outcome [2],[3],[4],[5],[6].
The first MVR at our institution was carried out in 1963. Now looking back at an experience of more than 30 years, we have reviewed our early and late results in this high-risk subgroup.
Material and methods
Patients
Since 1963, 2316 patients have undergone MVR at our institution and 382 of them had severe pulmonary hypertension with a mean pulmonary artery pressure (PAP) of more than 50 mmHg. The follow-up, with an observation period of 3208 patient years and a mean of 8.4±0.2 years per patient, was 95% complete.
We used 336 mechanical and 46 biological prostheses for MVR. The type of valve implanted was chosen by the surgeon concerned depending on availability at the time of the operation. In the period 1963–1973 caged-ball, caged-disc and tilting-disc prostheses were used. Implantation of biological valves was begun in 1976, but because reoperations became more frequent due to valve degeneration, bioprostheses were subsequently used less and less. The indications for implantation of biological prostheses had been the absence of any thromboembolic event, normal sinus rhythm, and good left ventricular function. Since 1979, the implantation of double-leaflet mechanical prostheses has been preferred.
The mean patient age has constantly increased over time, from 43 years initially to 58 years now, which is a difference of 15 years. Furthermore we have been confronted by a higher rate of reoperations, which has risen from 3.2 to 22.6%, as a result of the degeneration of bioprosthetic valves implanted in the previous decade.
Over the years, no difference has been seen in mean pulmonary pressure and pulmonary vascular resistance (PVR). The cardiac index ranged between 2.1 and 2.4 l/min per qm. For patient data see Table 1 .
Operative technique
MVR was carried out after median sternotomy. Extracorporeal circulation was established via bicaval and aortic cannulation. At the first operations the femoral artery was used for arterial access. Mild general hypothermia was employed, lowering the patients’ temperature to an average of 28°C. The intracardial procedure was carried out under ventricular fibrillation with additional topical cooling. Since 1987 cristalloid cardioplegic solutions (Custodiol®) have been used. The bypass time was 49.6±1.3 min, and the cross-clamping time was 30.6±0.7 min (mean±S.E.M.).
Anticoagulation
All patients received phenprocoumon (Marcumar®) beginning on the first postoperative day, whenever the postoperative course was uncomplicated. In long-term ventilated patients heparin was given intravenously to prevent valve thrombosis or emboli. After extubation, oral anticoagulation overlapping the heparin treatment was initiated. The therapeutic goal was to maintain the prothrombin time at 15–25%. Today, all surviving patients with a mechanical valve are still on this treatment. In patients receiving a bioprosthesis, the initial 3 months of anticoagulation was followed by a daily dose of 100 mg acetylsalicylic acid [7].
Follow-up
A questionnaire was sent to the last known address of all patients whose records did not contain a date of death entry. They were asked to complete this questionnaire together with their physician. Attempts were made to find patients who failed to return their questionnaires by contacting central telephone information services, resident registration offices, medical insurance companies, and personal physicians. The follow-up of our study groups was 95% complete, representing a total of 3208 patient years, with 19 patients (5%) who could not be located registered as lost to follow-up. Owing to the retrospective character of the study, post mortem examinations of the valves were not carried out.
Definitions
As recommended, we used the standardised definitions in the Guidelines for Reporting Morbidity and Mortality after Cardiac Valvular Operations of the American Association for Thoracic and Cardiovascular Surgery [8].
Statistics
Actuarial curves for long-term survival and freedom from complications were calculated using the methods of Kaplan and Meier [9]. All statistical analyses were done using the computer programmes SPSS for Windows, Version 6.0, 1993 and Excel Version 5.0, 1995.
Results
Mortality
Compared with the operative risk of patients undergoing routine elective MVR with no previous heart operations or pulmonary hypertension—which is about 3.6% at our hospital—the overall early mortality rate of 10.5% (n=40) is high. Starting from a mortality rate of 13.7% in the 1960s and 1970s, we saw a significant decline in the late 1970s and 1980s to 8.6%, and a slight increase to 10.8% in the late 1980s and early 1990s.
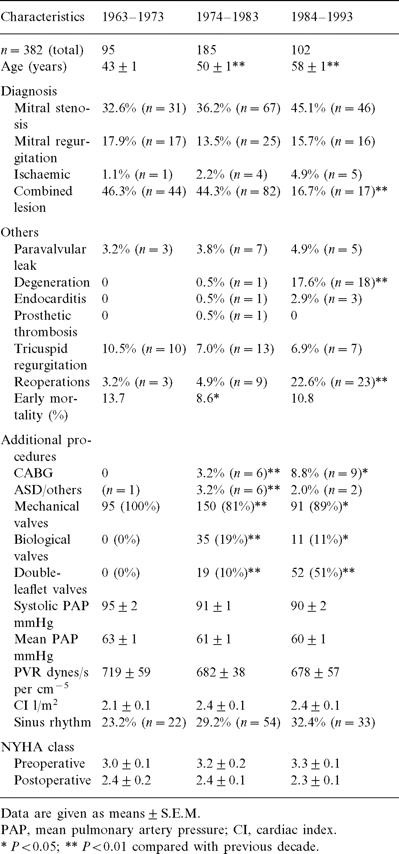
A consideration of the causes of early mortality reveals a number of interesting details. In the early 1960s and 1970s, factors unrelated to pulmonary hypertension, such as bleeding, embolism and septicaemia were more common causes of death (Fig. 1 ), as a result of the more blood-damaging cardiopulmonary bypass (CPB) techniques, less drug monitoring and the less effective antibiotics available at that time. Postoperative myocardial failure is a common cause of death because of borderline myocardial decompensation in severe pulmonary hypertension [3],[5],[10],[11],[12]. Despite the increase in patient-dependent risk factors (older age, reoperation), we did not observe any significant increase in the mortality rate, probably due to the improvements in CPB techniques, and the use of myocardial protection in the later decades.
Causes of early mortality after MVR in patients with severe pulmonary hypertension.
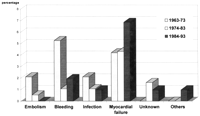
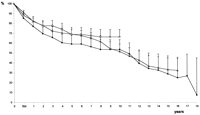
If we take a look at the actuarial survival curves after MVR we see a similar decline in survival rate over time in all patient groups, with a tendency towards better long-term results in the patients operated on later (Fig. 2 ). Taking patient age at the time of the operation into account—that is, comparing all operated patients with a matched control group of 1000 normal healthy individuals (data collection from insurance companies)—makes the results look different. In the first decade (1963–1973) we see a constant decline in survival over time which is less in the second decade (1974–1983). In the group of patients operated on between 1984 and 1993, a comparable decrease in survival 5 years postoperatively is found. The continuation of the curve showing a largely constant late survival rate to 10 years indicates that better long-term results are very likely (Fig. 3 ).
Actuarial survival after MVR in patients with severe pulmonary hypertension. Data shown as actuarial survival±S.E. – □ –, 1984–1993; – ▵ –, 1974–1983; – • –, 1963–1973.
Matched actuarial survival. Comparison of 1000 healthy controls versus patients with severe pulmonary hypertension.
Complication rates
The different linearised rates of such common complications as thromboembolism, bleeding, endocarditis and reoperation after MVR are shown in Table 2 , including the actuarial freedom of the already mentioned events.
Incidence of complications following MVR in patients with severe pulmonary hypertension: absolute numbers, linearised rates per patient year, and freedom from complications after 5, 10, 15 and 20 years
In the group operated on between 1974 and 1983, implantation of bioprostheses was very common (n=35; 19%; Table 1) and more thromboembolic events complicated the late postoperative course (P≪0.01). This fact is probably due to left atrial dilatation with onset of atrial fibrillation after valve degeneration.
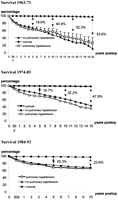
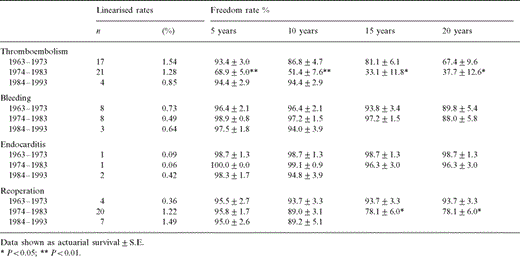
Quality of life depends on postoperative cardiac function and the incidence of complications. The event-free survival curves show that patients operated on between 1984 and 1993 achieved significantly better survival as compared with the patient groups operated on in previous decades (Fig. 4 ).
Event-free survival after MVR in patients with severe pulmonary hypertension. Data shown as actuarial survival±S.E. * P≪0.05; ** P≪0.01. – □ –, 1984–1993; – ▵ –, 1974–1983; – • –, 1963–1973.
In none of the survivors were any significant differences in cardiac function in terms of their postoperative New York Heart Association (NYHA) class were found (Table 1).
Pulmonary pressure and pulmonary vascular resistance
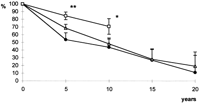
In 26 patients (16% of the survivors), invasive reinvestigation was done after a mean time of 7.1±1.0 years following MVR, because of a suspected onset of coronary disease, paravalvular leak, or other cardiac symptoms. According to the obtained data, mean pulmonary pressure drops significantly from 65±2 to 46±4 mmHg (P≪0.01), and PVR decreases significantly from 637±77 to 372±87 dynes/s per cm−5 (P≪0.05) (Fig. 5 ).
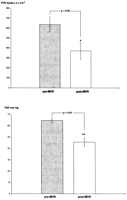
Long-term reduction of PVR and mean PAP after MVR. Data from 26 reinvestigated patients, mean pulmonary pressure PAP and PVR given as mean ±S.E.M. * P≪0.05; ** P≪0.01.
Discussion
As stated by Kirklin and Barratt-Boyes [13], whenever severe pulmonary hypertension is present preoperatively, it is usually the combined result of simple back pressure resulting from elevated left atrial pressure, and increased PVR [14]. The latter may have both a dynamic element, which is immediately reduced by lowering left atrial pressure, and an organic element from pulmonary vascular disease, which may or may not slowly regress after operation [2],[15],[16]. Thus, even severe pulmonary artery hypertension will very likely regress toward normal after MVR [17],[18]. This reduction often occurs soon after valve replacement, and thus seems related largely to the sudden drop in left atrial pressure and to a reversal of the severe spastic pulmonary vasoconstriction that accompanies left atrial hypertension in some patients [10],[11],[14]. Although this initial reduction rapidly appears in most patients, PVR sometimes continues to fall further in the months after the operation [3],[4],[6],[17].
In view of the results shown above it is necessary to ask what it is we have done in the past 30 years that has made mitral valve surgery safer for patients with severe pulmonary hypertension, despite the larger numbers of older, multimorbid and previously operated-on patients? We believe the following points to be of relevance.
Improvements in operative technique
Global myocardial ischaemic time has always been an incremental risk factor for death in the early phase after any cardiac operation. Generally, since the use of cardioplegic solutions results in better myocardial protection, the magnitude of mortality increase caused by prolonged aortic crossclamping times is less in the current era. Thus, operations on more severely damaged and weaker ventricles became possible [19],[20].
Further, improved CPB techniques and innovative operative techniques, such as the preservation of the subvalvular apparatus as reported by Hetzer et al. in 1983 [21] has led to better early results and long-term performance of the left ventricle as a result of less damage being done and ventricular geometry being preserved [22]. This technique was only feasible in about 30% of the cases in the last decade. This low rate is explained by the high number of reoperations among this patient group, where the whole subvalvular apparatus was excised in the previous procedure. In all cases where preservation of the subvalvular apparatus was possible, good functional results were achieved.
Since 1979 we have preferentially used the St. Jude Medical double-leaflet valve, which is associated with better haemodynamics and is therefore considered the ‘gold standard’ [23],[24],[25]. The percentage ratio of double-leaflet valves to disc prostheses between 1974 and 1983 is 10/71%. Between 1984 and 1993 it increased to 51/38%, and might have been an important factor in the good long-term performance of this group.
Since the introduction of aprotinin (trasylol®) [26], less bleeding, especially in redo cases, has led to a shorter ICU stay and therefore better recovery in the most recent years.
Improvements in perioperative patient care
Special online monitoring systems such as Swan–Ganz catheters have been developed, with the aim of detecting postoperative left ventricular dysfunction early, and measuring PAP continuously.
The possibility of being able to use different inotropic drugs, such as dopamine, dobutamine, adrenaline and noradrenaline made the treatment of postoperative ventricular dysfunction much more effective. Furthermore, the development of phosphodiesterase (PDE) inhibitors has resulted in a beneficial drop in mean PAP [10]—another positive effect. When mean pulmonary blood pressure remains elevated after MVR, drugs known from paediatric intensive care in the treatment of pulmonary hypertension, such as prostaglandin E2 (Flolan®) have recently been used successfully at our hospital; however we have no experience with nitric oxide inhalation [12].
Improvements in long-term postoperative care
Drug therapy for cardiac insufficiency became more effective when it was found that angiotensin-converting-enzyme (ACE) inhibitors can be used for that indication as well.
Self-monitoring of anticoagulation by the patient might improve late survival by reducing postoperative thromboembolic events and anticoagulant-related haemorrhage as a result of more accurate phenprocoumon (Marcumar®) dosing.
On the basis of our results we conclude that severe pulmonary hypertension is still an important preoperative risk factor, as reflected by our early mortality rates, but that it has no influence on late survival.
Despite an increase in risk factors in our patient population we have achieved significant better results over the years, and these have been consolidated over the last 10 years.
Appendix A. Conference discussion
Dr H. Borst (Munich, Germany): This is a rather remarkable study covering so many patients over a very long time. I just have one question. Your patients had pulmonary hypertension of 600 dynes, plus or minus. All of us in here probably would operate on such patients and feel fairly confident about it. However, I wonder whether you have been looking at some of the patients who really had a high resistance. The question of whether to operate or not only comes by the time the patient has a resistance of 1000 or 1200. So maybe you have a nice spin-off of your study telling us a little more about those high-risk cases.
Dr R.A. Cesnjevar: According to the literature, I think this is the greatest population of pulmonary hypertensive patients in a study up to now, and all patients underwent right heart catheterisation before the operation, so the pressures and resistances were measured and calculated afterwards. A lot of studies have been done about the drop of PVR immediately after cardiac surgery. And if you talk about conditions of 8–12 Wood units, the surgeons operating on patients with congenital heart disease get shocked, but it’s totally different in the adult and in mitral valve disease. Elevation of PVR results from the simple back pressure from the left atrium to the pulmonary veins, and there the elevated resistance is mostly caused by a spastic contraction of the pulmonary vessels. So a lot of it is reversible.
Dr H. Borst: Excuse me, but I didn’t really ask you that. I asked whether you identified patients with extremely high resistance who all died, is there a group of impossible patients in your collective?
Dr R.A. Cesnjevar: All right. To go over 1200 dynes makes a very bad survival, nearly 100% of them died.
Dr H. Borst: Over a thousand? Well, that’s very important information. I don’t think everybody would agree with that, but it’s certainly a very, very sorrowful group of patients.
Dr T. Kaul (Birmingham, Alabama): My published series on MVR in extreme pulmonary hypertension supports your finding and your conclusions. Over a 10-year study period, I had 30 patients with extreme pulmonary hypertension, with mean pulmonary artery systolic pressure of 118 mmHg, and PVR ranging over 10–35 Wood units. Hospital mortality was 5%, which was slightly higher than the remaining patients undergoing mitral valve surgery; but the 10-year survival was no different. After 5½ years, the haemodynamic studies showed a transpulmonary gradient reduced by 63.8%, left atrial pressure reduced by 53%, and the pulmonary artery mean pressure reduced by 58% [17].
The pulmonary hypertension in mitral valve disease develops in response to the raised left atrial pressure. There are two elements: (1) a reactive element, and (2) a structural or an organic element. The reactive element is known to decrease, as shown by Dr Dalen, Dr Zenner and myself, immediately after mitral valve surgery. The organic element is because of medial hypertrophy or intimal fibrosis in the pulmonary arterioles. Unlike congenital heart disease, these changes do not progress beyond grade III a–b changes described by Harris and Heath. In acquired mitral valve disease, plexiform lesions and arterialisation of elastic lamina fail to develop and the structural changes are reversible.
There is no direct or linear correlation between PVR and the medial hypertrophy. But as you said, and all others have known, and we have also demonstrated, pulmonary vascular resistance reduces after mitral valve surgery due to a gradual reversal of changes in pulmonary arterioles. That’s why the patients recover. Of course, they do need better postoperative care. We always take patient-related variables into account, but we do not take into account the care actually put into these patients. Your excellent results reflect the efforts you have put into this series.
Dr R.A. Cesnjevar: I think that it is important to identify those patients where the pulmonary changes are not reversible because of structural changes in the pulmonary capillary bed.
Dr F. Ciulli (Sheffield, UK): As always, in this type of study, results have to be looked at, in a multivariate fashion. Just talking about pulmonary hypertension is probably not enough to be able to identify the group of patients that will not survive this operation. In your experience, do you have an idea of an algorithm by which you could identify patients who will not benefit from including data such as dilatation of the right ventricle, tricuspid regurgitation, patients who have associated chronic obstructive airways disease? What patients should we be turning down for this type of surgery?
Dr R.A. Cesnjevar: At least from the data I have, I can say that tricuspid regurgitation is no reason for not accepting a patient for the operation. Chronic obstructive pulmonary disease—I haven’t looked at this rare condition in our mitral valve recipients. Just the other factors, like age, diabetes and so on, despite age they have no real influence in those patients. But chronic obstructive pulmonary disease, and other lung diseases—I didn’t look after that, sorry.
Dr L. Guvendik (Hull, UK): Did you look at the aetiology of mitral valve disease in these patients? Because mitral valve regurgitation with pulmonary hypertension is a different disease than mitral stenosis with pulmonary hypertension. Was there any difference.
Dr R.A. Cesnjevar: I agree. It’s a mixed study and the lesions are different. It’s about between 15 and 20% of mitral valve incompetence included in the study in all groups with no difference among the two groups and the three decades. So that’s why we didn’t divide it. So it made no big difference, no statistical significant difference.
Dr R. Landymore (Riyadh, Saudi Arabia): A number of your patients did not survive the operation. As a result of your experience with this group of patients, do you look more closely at the reactivity of the pulmonary vasculature? At our institution, we see many patients with end-stage valvular heart disease and severe pulmonary hypertension. We routinely carry out an adenosine test to determine the reversibility of the pulmonary hypertension.
The second question is: Do you know if those patients who died had dilated right hearts? We have found that the combination of severe pulmonary hypertension, dilated right heart, and tricuspid insufficiency is a predictor of mortality by multivariate analysis.
Dr R.A. Cesnjevar: We’re on the way to convincing our cardiologists to investigate these, but it’s very difficult because it’s an independent department, so you cant’ tell them what they should do. I agree that dilated right ventricles increase the risk after the operation, but that’s a known fact, I think.
References
Author notes
Presented at the 11th Annual Meeting of the European Association for Cardio-thoracic Surgery, Copenhagen, Denmark, 28 September–1 October, 1997.




