-
PDF
- Split View
-
Views
-
Cite
Cite
Sebastian König, Johannes Leiner, Anne Nitsche, Konstantinos Mouratis, Carolin Schanner, Anett Sommerschuh, Gerhard Hindricks, Andreas Meier-Hellmann, Ralf Kuhlen, Andreas Bollmann, Patients’ preferences regarding the digital capturing of patient-reported outcomes: planning the future follow-up in a prospective heart failure registry, European Heart Journal - Digital Health, Volume 2, Issue 4, December 2021, Pages 695–698, https://doi.org/10.1093/ehjdh/ztab074
Close - Share Icon Share
Abstract
Digital health technologies have the potential to improve patient care sustainably. A digital capturing of patient-reported outcome measures (PROMs) could facilitate patients’ surveillance and endpoint assessment within clinical trials especially in heart failure (HF) patients. However, data regarding the availability of digital infrastructure and patients’ willingness to use digital health solutions are scarce. Therefore, we conducted a survey as part of a digital-based HF registry.
The Helios Heart registry (H2-registry) has been introduced as a prospective registry being based on digitally augmented processes throughout the whole trial conduction from patients’ selection to data collection and follow-up (FU). Patient-reported outcome measures are captured paper-based at recruitment, but patients are offered two digital solutions for FU. Overall, 125 patients (mean age 67.8 years, 34.4% female) were included in the single-centre run-in phase of 16 weeks. Of them, 52.0% were not interested in any digital contact as part of the FU. If digital PROM capturing was conceivable, a web-based solution (70.0%) was preferred to an application-based approach (30.0%). Discrepancies occurred regarding the availability of email accounts and smartphones. Patients in the non-digital group were older (72.0 years vs. 63.2 years, P < 0.01) and more frequently female (female sex, non-digital vs. digital group: 47.7% vs. 20.0%, P < 0.01).
Our survey illustrated difficulties of implementing a digital FU to record PROMs in a contemporary HF cohort in particular among older patients. Further research is required to specify reasons in case of patients’ unwillingness and to better tailor digital health solutions to patients’ specific needs.
Rising patient numbers and emerging therapeutic options related to heart failure (HF) are calling for pragmatic and efficient strategies both in the context of clinical practice as well as scientific research to improve future patient care.1 Digital healthcare solutions are evolving and will make relevant contributions in this field.2 Mobile health (mHealth) technologies were already shown to be feasible when used for capturing health data in HF patients.3 Moreover, mHealth-guided interventions with remote monitoring turned out to improve clinical outcomes.4 Capturing patient-reported outcome measures (PROMs) on a large scale using mHealth technologies would be an obvious next step that would facilitate patients’ surveillance and endpoint assessment. This is especially true when considering problems with classic PROM acquisition like administrative and personnel burden regarding data collection from a professionals’ as well as literacy issues from a patients’ perspective.5 In this light, Campbell et al.6 reported on the availability of digital infrastructure among 50 HF patients. There, each 84% of participants stated to own a smartphone and to have internet at home. However, data regarding patients’ willingness and preferred modes of digital interaction with health care providers is scarce. Therefore, we present data from a survey aiming to answer this relevant question as a sub-project of a newly introduced, digital-based HF registry.
We initiated the Helios Heart registry (H2-registry) as a prospective observational registry across Germany in March 2021 with the explicit focus on pursuing a digital approach throughout the whole trial conduction. Eligible patients according to in- and exclusion criteria (for detailed information see clinicaltrials.gov: NCT04844944) are randomly selected by automated algorithms utilizing data from the hospital information system (HIS) on a daily basis followed by a subsequent verification of eligibility and obtaining informed consent by the trial investigators. Captured variables and endpoints are based on the validated International Consortium for Health Outcomes Measurement (ICHOM) standard set for HF patients.7 Numerous baseline characteristics are extracted automatically by the implementation of internally validated algorithms from the HIS. Patient-reported outcome measures (adapted from ICHOM: PROMIS Global Health 10, PHQ-2, KCCQ-12) are captured paper-based during the index hospitalization but are processed via an optical character recognition for automated integration into the study database afterwards. Regarding the PROM gathering during follow-up (FU), two digital solutions (application for smartphones/tablets, designated website) are offered to patients including a short survey assessing digital capabilities and patients’ motivation.
Overall, 125 HF patients (mean age 67.8 years, 34.4% female) were investigated in the single-centre (Heart Center Leipzig) run-in phase of the future planned multicentre registry for this interim analysis after 16 weeks of recruitment. Of them, 52.0% of patients were not interested in an ongoing PROM assessment via any digital solution but preferred classic FU via mail or telephone interviews. More than half of the patients indicated that they either do not have an email address or are not willing to provide it for study purposes. The majority of those being open to a digital FU (48.0%) preferred a web-based completion of PROM questionnaires (70.0%) as opposed to 30.0% favouring an application-based approach. Interestingly, seven patients specified that they could imagine using a smartphone application for the completion of questionnaires without providing an email address. Of them, two patients did not want to give their email address but own a smartphone, two patients use a smartphone but stated not to have an email client and three patients indicated, they could use a family member’s device for this purpose. Results regarding the willingness to participate in digital FU are illustrated in Figure 1. Patients who refused to use digital solutions for PROMs were older (72.0 years vs. 63.2 years, P < 0.01) and more likely to be women (female sex in non-digital vs. digital group: 47.7% vs. 20.0%, P < 0.01). Table 1 lists survey responses stratified for different age groups.
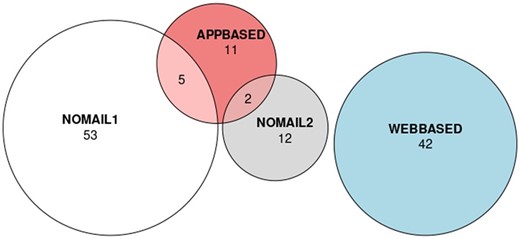
Results of the survey on willingness for digital completion of PROMs. APPBASED, Application-based PROM completion preferred (via smartphone/tablet); NOMAIL1, No email address; NOMAIL2, Email address available but no use desired for purpose of PROM completion; WEBASED, Web-based PROM completion preferred (via browser).
Results of the survey on willingness for digital completion of PROMs by age groups
| Age group (years) . | No email address . | Email address available but no use desired for this purpose . | Web-based PROM completion preferred (via browser) . | Application-based PROM completion preferred (via smartphone/tablet) . |
|---|---|---|---|---|
| All age groups (n = 125) | 58 (46.4%) | 14 (11.2%) | 42 (33.6%) | 18 (14.4%) |
| <55 (n = 15) | 3 (20.0%) | 3 (20.0%) | 8 (53.3%) | 2 (13.3%) |
| 55–64 (n = 31) | 13 (41.9%) | 4 (12.9%) | 10 (32.3%) | 8 (25.8%) |
| 65–74 (n = 38) | 12 (31.6%) | 4 (10.5%) | 18 (47.4%) | 6 (15.8%) |
| ≥75 (n = 41) | 30 (73.2%) | 3 (7.3%) | 6 (14.6%) | 2 (4.9%) |
| Age group (years) . | No email address . | Email address available but no use desired for this purpose . | Web-based PROM completion preferred (via browser) . | Application-based PROM completion preferred (via smartphone/tablet) . |
|---|---|---|---|---|
| All age groups (n = 125) | 58 (46.4%) | 14 (11.2%) | 42 (33.6%) | 18 (14.4%) |
| <55 (n = 15) | 3 (20.0%) | 3 (20.0%) | 8 (53.3%) | 2 (13.3%) |
| 55–64 (n = 31) | 13 (41.9%) | 4 (12.9%) | 10 (32.3%) | 8 (25.8%) |
| 65–74 (n = 38) | 12 (31.6%) | 4 (10.5%) | 18 (47.4%) | 6 (15.8%) |
| ≥75 (n = 41) | 30 (73.2%) | 3 (7.3%) | 6 (14.6%) | 2 (4.9%) |
Percentages can add to >100% since multiple answers are possible.
PROM, patient-reported outcome measure.
Results of the survey on willingness for digital completion of PROMs by age groups
| Age group (years) . | No email address . | Email address available but no use desired for this purpose . | Web-based PROM completion preferred (via browser) . | Application-based PROM completion preferred (via smartphone/tablet) . |
|---|---|---|---|---|
| All age groups (n = 125) | 58 (46.4%) | 14 (11.2%) | 42 (33.6%) | 18 (14.4%) |
| <55 (n = 15) | 3 (20.0%) | 3 (20.0%) | 8 (53.3%) | 2 (13.3%) |
| 55–64 (n = 31) | 13 (41.9%) | 4 (12.9%) | 10 (32.3%) | 8 (25.8%) |
| 65–74 (n = 38) | 12 (31.6%) | 4 (10.5%) | 18 (47.4%) | 6 (15.8%) |
| ≥75 (n = 41) | 30 (73.2%) | 3 (7.3%) | 6 (14.6%) | 2 (4.9%) |
| Age group (years) . | No email address . | Email address available but no use desired for this purpose . | Web-based PROM completion preferred (via browser) . | Application-based PROM completion preferred (via smartphone/tablet) . |
|---|---|---|---|---|
| All age groups (n = 125) | 58 (46.4%) | 14 (11.2%) | 42 (33.6%) | 18 (14.4%) |
| <55 (n = 15) | 3 (20.0%) | 3 (20.0%) | 8 (53.3%) | 2 (13.3%) |
| 55–64 (n = 31) | 13 (41.9%) | 4 (12.9%) | 10 (32.3%) | 8 (25.8%) |
| 65–74 (n = 38) | 12 (31.6%) | 4 (10.5%) | 18 (47.4%) | 6 (15.8%) |
| ≥75 (n = 41) | 30 (73.2%) | 3 (7.3%) | 6 (14.6%) | 2 (4.9%) |
Percentages can add to >100% since multiple answers are possible.
PROM, patient-reported outcome measure.
Compared to the analysis of Campbell et al.,6 less patients reported to have access to an email address despite a similar age distribution. Overall, the willingness to use digital applications appears to be lower in our cohort. Regional differences could be one influencing factor to those findings with possible implications regarding generalizability. However, even though our cohort only recruited patients from Germany, the German population tends to be at or above average in terms of quantitative internet use as an indicator of digital capabilities in a European comparison.8 Moreover, in our analysis, patients were specifically asked for their intention to participate in a certain clinical investigation. It remains unclear whether all patients who specified to have an email address or smartphone are willing to use it for medical study purposes. It is noteworthy that the observation of patients having access to a smartphone but do not use email was also made in the survey by Campbell et al.6 Nevertheless, some difficulties may occur regarding the provision of questionnaires when an email address is not available. Possible solutions may include sending QR codes with personalized login data via mail. Another factor that could potentially influence patients’ response regarding a digital PROM FU may be the analogue PROM acquisition during onboarding. Directly introducing a mHealth solution may lead to an increased willingness to use this tool in the future as well. Finally, it needs to be pointed out that the current approach addressed PROM acquisition only. Whether or not the add-on of educational material and services (e.g., medication management, teleconsultation) would affect patients’ beliefs regarding the app use needs to be determined.
Since PROMs are critical for patient-centred healthcare and have an impact on both therapy adherence and outcome, further efforts are required to enable their efficient collection.9,10 Our survey illustrated limits of implementing a digital PROM FU in particular among older HF patients. However, there was a relevant proportion of participants who declared their willingness to use mHealth solutions, including patients not regularly implementing digital technologies in their everyday life. Possible implications for the future would therefore be to introduce programmes to improve patients’ digital literacy, in the best case connected to the professional health care system (e.g. HF care programmes at general practitioners and cardiologists, HF ambulances), and to introduce specifically designed mHealth applications in order to both improve patients’ capabilities as well as to reduce inhibitions and distrust associated with mHealth. Doing so, the implementation of PROMs on a broader basis could be facilitated, which may improve future patient care.
Funding
The execution of the underlying Helios Heart registry has been funded by AstraZeneca Germany.
Conflict of interest: There has been a funding of the underlying Helios Heart registry by AstraZeneca Germany. No author has received any personal payment or alternative remuneration in this regard.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Eurostat. Digital society statistics at regional level.
Author notes
The first two authors contributed equally to this manuscript.




