-
PDF
- Split View
-
Views
-
Cite
Cite
Seung-Jun Lee, Jae Hong Joo, Sohee Park, Choongki Kim, Dong-Woo Choi, Yong-Joon Lee, Sung-Jin Hong, Chul-Min Ahn, Jung-Sun Kim, Byeong-Keuk Kim, Young-Guk Ko, Donghoon Choi, Yangsoo Jang, Chung-Mo Nam, Myeong-Ki Hong, Combination therapy with moderate-intensity atorvastatin and ezetimibe vs. high-intensity atorvastatin monotherapy in patients treated with percutaneous coronary intervention in practice: assessing RACING generalizability, European Heart Journal - Cardiovascular Pharmacotherapy, Volume 10, Issue 8, December 2024, Pages 676–685, https://doi.org/10.1093/ehjcvp/pvad083
Close - Share Icon Share
Abstract
Using rosuvastatin, the RACING (randomized comparison of efficacy and safety of lipid-lowering with statin monotherapy versus statin/ezetimibe combination for high-risk cardiovascular diseases) trial showed the beneficial effects of combining moderate-intensity statin with ezetimibe compared with high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease. This study investigated whether the beneficial effects of combination lipid-lowering therapy extend to patients treated with atorvastatin, not rosuvastatin, in daily clinical practice.
Using stabilized inverse probability of treatment weighting, a total of 31 993 patients who were prescribed atorvastatin after drug-eluting stent (DES) implantation were identified from a nationwide cohort database: 6215 patients with atorvastatin 20 mg plus ezetimibe 10 mg (combination lipid-lowering therapy) and 25 778 patients with atorvastatin 40–80 mg monotherapy. The primary endpoint was the 3-year composite of cardiovascular death, myocardial infarction, coronary artery revascularization, hospitalization for heart failure treatment, or non-fatal stroke in accordance with the RACING trial design. Combination lipid-lowering therapy was associated with a lower incidence of the primary endpoint (12.9% vs. 15.1% in high-intensity atorvastatin monotherapy; hazard ratio [HR] 0.81, 95% confidence interval [CI] 0.74–0.88, P < 0.001). Compared with high-intensity atorvastatin monotherapy, combination lipid-lowering therapy was also significantly associated with lower rates of statin discontinuation (10.0% vs. 8.4%, HR 0.81, 95% CI 0.73–0.90, P < 0.001) and new-onset diabetes requiring medication (8.8% vs. 7.0%, HR 0.80, 95% CI 0.70–0.92, P = 0.002).
In clinical practice, a combined lipid-lowering approach utilizing ezetimibe and moderate-intensity atorvastatin was correlated with favourable clinical outcomes, drug compliance, and a reduced incidence of new-onset diabetes requiring medications in patients treated with DES implantation.
Trial registration: ClinicalTrial.gov (NCT04715594).
Introduction
For patients who have undergone percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation, stringent dyslipidaemia control is of paramount importance to mitigate the risk of subsequent adverse cardiovascular events.1,2 Although the use of high-intensity statins is strongly recommended to achieve the target low-density lipoprotein cholesterol (LDL-C) level suggested by international guidelines, concerns about drug-related side effects lead to substantial underuse of guideline-recommended treatment in practice.3,4 Instead of obligatorily beginning with high-intensity statins and considering the addition of ezetimibe only after adverse events or patient intolerance occurs, beginning with combination lipid-lowering therapy might be a reasonable therapeutic approach.5 The randomized RACING trial (randomized comparison of efficacy and safety of lipid-lowering with statin monotherapy versus statin/ezetimibe combination for high-risk cardiovascular diseases) corroborated that idea by demonstrating that the combination of a moderate-intensity statin (rosuvastatin) and ezetimibe was non-inferior to high-intensity rosuvastatin monotherapy in terms of 3-year adverse cardiovascular events in patients with atherosclerotic cardiovascular disease (ASCVD).6
In clinical practice, atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor capable of providing moderate- to high-intensity lipid-lowering effects depending on the dosage (along with rosuvastatin), is the most common medication prescribed for patients who have undergone PCI.7 However, the therapeutic benefit of combining moderate-intensity atorvastatin with ezetimibe vs. that with high-intensity atorvastatin monotherapy in patients who have undergone PCI has not been identified. In this study, we investigated whether the results of the RACING trial can be extended to patients treated with atorvastatin in daily clinical practice.
Methods
Study design and data
For this study, we conducted a nationwide retrospective analysis of the National Health Claims Database administered by the National Health Insurance Service (NHIS) of Korea. This comprehensive database contains detailed information about medical costs, precise prescription details such as medication dosage and quantity, and medical history classified as International Classification of Diseases, Tenth Revision codes.8 The NHIS, managed by the Korean government, has a mandatory subscription rate of 97.1% among the total Korean population. The NHIS database also contains data for the remaining 2.9% of the population classified as medical aid recipients, thereby providing a comprehensive representation of the entire Korean population.9 Because personal information was strictly de-identified during cohort establishment, according to the guidelines of the Korean Health Insurance Review and Assessment Service, the requirement for individual informed consent was waived. Causes of death were provided by the National Institute of Statistics of Korea. This study was approved by the Institutional Review Board of our institute. This study was registered at ClinicalTrial.gov (NCT04715594).
Cohort design and study population
Among the 52 million Korean citizens included in the NHIS database, we identified 273 670 adult patients (≥20 years old) who underwent DES implantation between January 2005 and December 2015 (CONNECT DES cohort registry).8 To align with the eligibility criteria of the RACING trial, we excluded patients with chronic liver disease (n = 15 648), solid organ transplant recipients (n = 1239), and people who were or might become pregnant (n = 199).10 To broaden the applicability and generalizability of the findings from the randomized RACING trial, which compared the therapeutic effects of daily rosuvastatin 10 mg plus ezetimibe 10 mg with those of daily high-intensity rosuvastatin 20 mg monotherapy over a 3-year period in 3780 patients with documented ASCVD,6 an equivalent dose of atorvastatin was used as the primary inclusion criterion in this study: atorvastatin 20 mg plus ezetimibe 10 mg for the combination lipid-lowering therapy and atorvastatin 40–80 mg for high-intensity statin monotherapy. Therefore, we excluded patients treated with statins other than atorvastatin (n = 113 216), those who did not meet the statin intensity criteria specified in the RACING trial (n = 94 828), and patients who did not receive any statin treatment after their PCI (n = 6216). Additionally, patients who died within 7 days of PCI (n = 4823) or had missing covariate data (n = 5535) were also excluded from the study.10 Consequently, this study included the remaining 31 966 patients with DES implantation who were treated with daily atorvastatin 20 mg plus ezetimibe 10 mg (n = 6164) or daily atorvastatin 40–80 mg (n = 25 802) between January 2016 and December 2018 (Figure 1). Before 2016, the use of ezetimibe was covered by insurance criteria in Korea only for patients who did not achieve sufficient LDL-C reduction on the high-intensity or maximum statin dose. Therefore, the combination of ezetimibe with a moderate-intensity statin, which we aimed to validate in this study, was rarely applied in clinical practice before 2016.10 The details of the trial protocol are compared with those of the RACING trial in Supplementary material online Table S1.
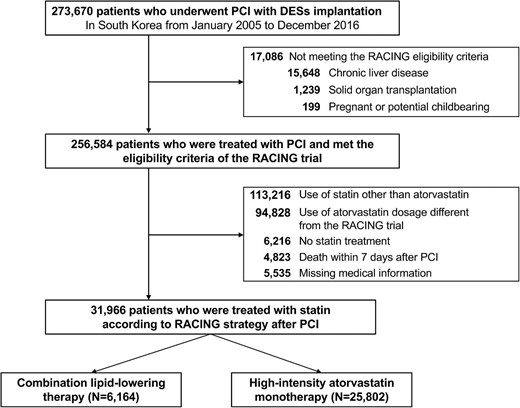
Flow chart of the study population. DES, drug-eluting stent; PCI, percutaneous coronary intervention; RACING, randomized comparison of efficacy and safety of lipid-lowering with statin monotherapy vs. statin/ezetimibe combination for high-risk cardiovascular diseases.
Study outcomes
In line with the randomized RACING trial,6 the primary endpoint was a composite of cardiovascular death, myocardial infarction (MI), coronary artery revascularization, hospitalization for heart failure, and non-fatal stroke occurring during 3 years of follow-up from the first prescription of study medication between January 2016 and December 2018.10 To determine the occurrence of cardiovascular death, death certificates provided by the National Statistical Office of Korea were analysed, with a 92% accuracy rate in attributing the cause of death.9–11 For classification as cardiovascular death, a death certificate had to indicate at least one diagnosis related to cardiovascular conditions such as acute MI, stroke, heart failure, or sudden cardiac death. MI was defined by the International Classification of Diseases, Tenth Revision codes corresponding to acute MI, along with meeting one or more of the following criteria: (1) concurrent claims for coronary angiography, (2) admission through the emergency department, or (3) cardiac biomarkers tested more than four times.10 A detailed description of each efficacy endpoint can be found in Supplementary material online Table S2. The secondary safety endpoints were (1) statin discontinuation for ˃180 days and (2) the incidence of adverse clinical endpoints: new-onset diabetes mellitus requiring medication, rhabdomyolysis, gall-bladder disease requiring cholecystectomy, and a new diagnosis of cancer. A comprehensive description of each safety endpoint is presented in Supplementary material online Table S3.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation, and dichotomous variables are presented as the frequency and percentage. To mitigate the effects of confounding bias, we used inverse probability of treatment weighting (IPTW) by calculating propensity scores with a logistic regression of age, sex, history of comorbidities and medications, and year of PCI (Supplementary material online Table S4). The IPTW was further stabilized by multiplying it with the marginal probability of receiving each treatment. Standardized mean difference and Kernel density plots were used to determine the effect size difference between the groups for baseline comorbidities and medications. Standardized mean difference values exceeding 0.2 indicated a potential imbalance between the groups. To mitigate potential residual bias, the IPTW model was additionally adjusted for multivariate variables, including age, sex, prior statin therapy, previous medication use, and year of PCI. Cumulative incidence and the occurrence rates of the primary and secondary endpoints during the follow-up period were plotted using the Kaplan–Meier method. To assess the adjusted hazard ratio (HR) for each clinical endpoint, a Cox proportional hazard regression model was used. In analysing the incidences of cardiovascular death, MI, and hospitalization due to heart failure, cause-specific hazard models were used to account for death as a competing risk. Sensitivity analyses were conducted to validate the robustness of the main findings for (1) baseline characteristic, (2) propensity-score-matched population, and (3) safety population after exclusion of the patients who were not given the initial statin therapy during the study period. A two-sided P-value <0.05 was considered significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.0 (The R Foundation, www.R-project.org).
Results
Both the combination lipid-lowering therapy group and the high-intensity atorvastatin monotherapy group had an average follow-up period of 2.9 ± 0.3 years. Baseline demographics, medical histories, and procedural information for the cohort population before and after stabilized IPTW are presented in Table 1. All baseline characteristics were well balanced between the two groups after stabilized IPTW (all standardized mean differences <0.1, Supplementary material online Figures S1 and S2). Consequently, the study participants (n = 31 993) fulfilled the eligibility criteria of the RACING trial and received either moderate-intensity atorvastatin with ezetimibe combination therapy (n = 6 215) or high-intensity atorvastatin monotherapy (n = 25 778). Among the high-intensity atorvastatin monotherapy group, daily atorvastatin 80 mg was administered to 3466 patients throughout the study period, and 22 312 received daily atorvastatin 40 mg. Changes in statin intensity during the 3-year study period are illustrated in Supplementary material online Figure S3. In the combination lipid-lowering therapy group, 5314 patients (85.5%) continued to receive the initial combination therapy of moderate-intensity atorvastatin and ezetimibe, and in the statin monotherapy group, 19 282 patients (74.8%) continued to have high-intensity atorvastatin monotherapy (P < 0.001). The cumulative incidence and relative hazards of the primary endpoints are presented in Table 2 and Figure 2. The combination lipid-lowering therapy group showed a significantly lower incidence rate for the primary endpoint than the high-intensity atorvastatin monotherapy group (12.9% vs. 15.1%, respectively, HR 0.81, 95% confidence interval [CI] 0.74–0.88, P < 0.001, number needed to treat [NNT] = 45). The cumulative incidences of each clinical endpoint are also presented in Table 2. During the 3-year study period, combination lipid-lowering therapy was associated with a significantly lower occurrence of cardiovascular death (NNT = 109), all-cause death (NNT = 122), MI (NNT = 210), coronary revascularization (NNT = 167), and non-fatal stroke (NNT = 130), compared with high-intensity atorvastatin monotherapy (all P < 0.05). Subgroup analyses consistently demonstrated the therapeutic benefits of combination lipid-lowering therapy vs. high-intensity atorvastatin monotherapy for the primary endpoint (Figure 3). The 3-year occurrence of each safety endpoint is presented in Table 3. Combination lipid-lowering therapy was associated with a significantly lower rate of statin discontinuation (8.4% vs. 10.0% in high-intensity atorvastatin monotherapy, HR 0.81, 95% CI 0.73–0.90, P < 0.001, NNT = 62) and new-onset diabetes requiring medication (7.0% vs. 8.8% in high-intensity atorvastatin monotherapy, HR 0.80, 95% CI 0.70–0.92, P = 0.002, NNT = 57). Sensitivity analyses for the propensity-score-matched population (Supplementary material online Table S5) and safety population (Supplementary material online Table S6) demonstrated consistent findings.
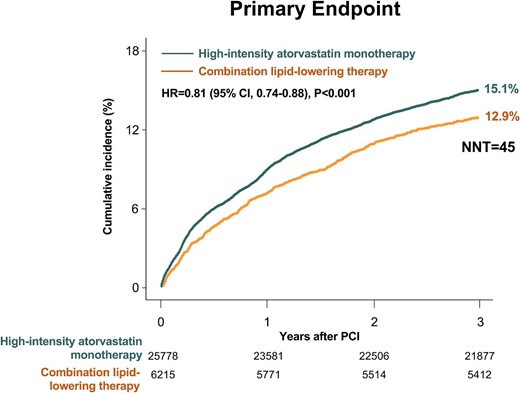
Time-to-event curves for the primary endpoint. The cumulative incidence of the primary endpoint across 3 years compared between the two treatment strategies. CI, confidence interval; HR, hazard ratio; PCI, percutaneous coronary intervention; NNT, number needed to treat.
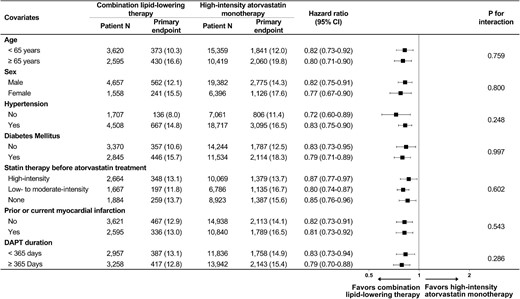
Subgroup analysis regarding the primary endpoint. Numbers and percentages show the number of patients at risk and the incidence of the primary endpoint. CI, confidence interval; DAPT, dual antiplatelet therapy.
| . | Before stabilized IPTW (N = 31 966) . | After stabilized IPTW (N = 31 993) . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Combination lipid-lowering therapy (N = 6164) . | High-intensity atorvastatin monotherapy (N = 25 802) . | SMD . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | SMD . |
| Age, years | 61.9 ± 10.9 | 62.9 ± 11.3 | 0.087 | 62.8 ± 11.0 | 62.7 ± 11.2 | 0.007 |
| Female | 1709 (27.7) | 6229 (24.1) | 0.082 | 1558 (25.1) | 6396 (24.8) | 0.006 |
| Comorbidity | ||||||
| Hypertension | 4730 (76.7) | 18 509 (71.7) | 0.115 | 4508 (72.5) | 18 717 (72.6) | 0.002 |
| Chronic kidney disease with severe renal impairmenta | 401 (6.5) | 1427 (5.5) | 0.041 | 392 (6.3) | 1485 (5.8) | 0.023 |
| Diabetes mellitus | 3033 (49.2) | 11 273 (43.7) | 0.111 | 2845 (45.8) | 11 534 (44.7) | 0.021 |
| Statin therapy before atorvastatin treatmentb | ||||||
| High-intensity statin | 1285 (20.8) | 11 098 (43.0) | 0.784 | 2664 (42.9) | 10 069 (39.1) | 0.097 |
| Low- to moderate-intensity statin | 3400 (55.2) | 5208 (20.2) | 1667 (26.8) | 6786 (26.3) | ||
| None | 1479 (24.0) | 9496 (36.8) | 1884 (30.3) | 8923 (34.6) | ||
| Charlson comorbidity index | 3.5 ± 2.3 | 3.3 ± 2.3 | 0.068 | 3.4 ± 2.3 | 3.3 ± 2.3 | 0.020 |
| Atherosclerotic cardiovascular disease | ||||||
| Prior or current myocardial infarction | 2588 (42.0) | 10 872 (42.1) | 0.003 | 2595 (41.7) | 10 840 (42.1) | 0.006 |
| Prior coronary bypass graft surgery | 103 (1.7) | 336 (1.3) | 0.030 | 101 (1.6) | 362 (1.4) | 0.018 |
| Prior ischaemic stroke | 969 (15.7) | 4488 (17.4) | 0.045 | 1108 (17.8) | 4413 (17.1) | 0.019 |
| Peripheral artery disease | 399 (6.5) | 1349 (5.2) | 0.053 | 350 (5.6) | 1414 (5.5) | 0.007 |
| Medication before PCI | ||||||
| Aspirin | 4675 (75.8) | 15 932 (61.8) | 0.308 | 4288 (69.0) | 16 669 (64.7) | 0.092 |
| Clopidogrel | 3808 (61.8) | 12 005 (46.5) | 0.310 | 3172 (51.0) | 12 767 (49.5) | 0.030 |
| β-Blocker | 3222 (52.3) | 11 384 (44.1) | 0.164 | 3023 (48.6) | 11 827 (45.9) | 0.055 |
| RAAS blockade | 2966 (48.1) | 10 544 (40.9) | 0.146 | 2744 (44.2) | 10 930 (42.4) | 0.035 |
| Procedural information | ||||||
| Number of stents | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.023 | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.043 |
| Type of drug-eluting stent | ||||||
| First generationc | 1161 (18.8) | 3120 (12.1) | 0.187 | 781 (12.6) | 3435 (13.3) | 0.022 |
| Next generation | 5003 (81.2) | 22 682 (87.9) | 5434 (87.4) | 22 343 (86.7) | ||
| Duration of dual antiplatelet therapy | ||||||
| <365 days | 2610 (42.3) | 12 004 (46.5) | 0.084 | 2957 (47.6) | 11 836 (45.9) | 0.033 |
| ≥365 days | 3554 (57.7) | 13 798 (53.5) | 3258 (52.4) | 13 942 (54.1) | ||
| Year of PCI | ||||||
| 2005–2009 | 1370 (22.2) | 3878 (15.0) | 0.251 | 991 (16.0) | 4226 (16.4) | 0.066 |
| 2010–2014 | 1799 (29.2) | 6322 (24.5) | 1401 (22.5) | 6667 (25.1) | ||
| 2015–2016 | 2995 (48.6) | 15 602 (60.5) | 3823 (51.5) | 15 085 (58.5) | ||
| . | Before stabilized IPTW (N = 31 966) . | After stabilized IPTW (N = 31 993) . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Combination lipid-lowering therapy (N = 6164) . | High-intensity atorvastatin monotherapy (N = 25 802) . | SMD . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | SMD . |
| Age, years | 61.9 ± 10.9 | 62.9 ± 11.3 | 0.087 | 62.8 ± 11.0 | 62.7 ± 11.2 | 0.007 |
| Female | 1709 (27.7) | 6229 (24.1) | 0.082 | 1558 (25.1) | 6396 (24.8) | 0.006 |
| Comorbidity | ||||||
| Hypertension | 4730 (76.7) | 18 509 (71.7) | 0.115 | 4508 (72.5) | 18 717 (72.6) | 0.002 |
| Chronic kidney disease with severe renal impairmenta | 401 (6.5) | 1427 (5.5) | 0.041 | 392 (6.3) | 1485 (5.8) | 0.023 |
| Diabetes mellitus | 3033 (49.2) | 11 273 (43.7) | 0.111 | 2845 (45.8) | 11 534 (44.7) | 0.021 |
| Statin therapy before atorvastatin treatmentb | ||||||
| High-intensity statin | 1285 (20.8) | 11 098 (43.0) | 0.784 | 2664 (42.9) | 10 069 (39.1) | 0.097 |
| Low- to moderate-intensity statin | 3400 (55.2) | 5208 (20.2) | 1667 (26.8) | 6786 (26.3) | ||
| None | 1479 (24.0) | 9496 (36.8) | 1884 (30.3) | 8923 (34.6) | ||
| Charlson comorbidity index | 3.5 ± 2.3 | 3.3 ± 2.3 | 0.068 | 3.4 ± 2.3 | 3.3 ± 2.3 | 0.020 |
| Atherosclerotic cardiovascular disease | ||||||
| Prior or current myocardial infarction | 2588 (42.0) | 10 872 (42.1) | 0.003 | 2595 (41.7) | 10 840 (42.1) | 0.006 |
| Prior coronary bypass graft surgery | 103 (1.7) | 336 (1.3) | 0.030 | 101 (1.6) | 362 (1.4) | 0.018 |
| Prior ischaemic stroke | 969 (15.7) | 4488 (17.4) | 0.045 | 1108 (17.8) | 4413 (17.1) | 0.019 |
| Peripheral artery disease | 399 (6.5) | 1349 (5.2) | 0.053 | 350 (5.6) | 1414 (5.5) | 0.007 |
| Medication before PCI | ||||||
| Aspirin | 4675 (75.8) | 15 932 (61.8) | 0.308 | 4288 (69.0) | 16 669 (64.7) | 0.092 |
| Clopidogrel | 3808 (61.8) | 12 005 (46.5) | 0.310 | 3172 (51.0) | 12 767 (49.5) | 0.030 |
| β-Blocker | 3222 (52.3) | 11 384 (44.1) | 0.164 | 3023 (48.6) | 11 827 (45.9) | 0.055 |
| RAAS blockade | 2966 (48.1) | 10 544 (40.9) | 0.146 | 2744 (44.2) | 10 930 (42.4) | 0.035 |
| Procedural information | ||||||
| Number of stents | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.023 | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.043 |
| Type of drug-eluting stent | ||||||
| First generationc | 1161 (18.8) | 3120 (12.1) | 0.187 | 781 (12.6) | 3435 (13.3) | 0.022 |
| Next generation | 5003 (81.2) | 22 682 (87.9) | 5434 (87.4) | 22 343 (86.7) | ||
| Duration of dual antiplatelet therapy | ||||||
| <365 days | 2610 (42.3) | 12 004 (46.5) | 0.084 | 2957 (47.6) | 11 836 (45.9) | 0.033 |
| ≥365 days | 3554 (57.7) | 13 798 (53.5) | 3258 (52.4) | 13 942 (54.1) | ||
| Year of PCI | ||||||
| 2005–2009 | 1370 (22.2) | 3878 (15.0) | 0.251 | 991 (16.0) | 4226 (16.4) | 0.066 |
| 2010–2014 | 1799 (29.2) | 6322 (24.5) | 1401 (22.5) | 6667 (25.1) | ||
| 2015–2016 | 2995 (48.6) | 15 602 (60.5) | 3823 (51.5) | 15 085 (58.5) | ||
Values are presented as the mean ± standard deviation or n (%).
IPTW, inverse probability of treatment weighting; SMD, standardized mean difference; PCI, percutaneous coronary intervention; RAAS, renin–angiotensin–aldosterone system.
Chronic kidney disease with advanced stage requiring intensive medical therapy and financial assistance from health insurance.
The intensity of prior statin treatment was categorized based on the 2013 American College of Cardiology/American Heart Association guideline for blood cholesterol treatment.
First-generation drug-eluting stent indicates Cypher and Taxus.
| . | Before stabilized IPTW (N = 31 966) . | After stabilized IPTW (N = 31 993) . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Combination lipid-lowering therapy (N = 6164) . | High-intensity atorvastatin monotherapy (N = 25 802) . | SMD . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | SMD . |
| Age, years | 61.9 ± 10.9 | 62.9 ± 11.3 | 0.087 | 62.8 ± 11.0 | 62.7 ± 11.2 | 0.007 |
| Female | 1709 (27.7) | 6229 (24.1) | 0.082 | 1558 (25.1) | 6396 (24.8) | 0.006 |
| Comorbidity | ||||||
| Hypertension | 4730 (76.7) | 18 509 (71.7) | 0.115 | 4508 (72.5) | 18 717 (72.6) | 0.002 |
| Chronic kidney disease with severe renal impairmenta | 401 (6.5) | 1427 (5.5) | 0.041 | 392 (6.3) | 1485 (5.8) | 0.023 |
| Diabetes mellitus | 3033 (49.2) | 11 273 (43.7) | 0.111 | 2845 (45.8) | 11 534 (44.7) | 0.021 |
| Statin therapy before atorvastatin treatmentb | ||||||
| High-intensity statin | 1285 (20.8) | 11 098 (43.0) | 0.784 | 2664 (42.9) | 10 069 (39.1) | 0.097 |
| Low- to moderate-intensity statin | 3400 (55.2) | 5208 (20.2) | 1667 (26.8) | 6786 (26.3) | ||
| None | 1479 (24.0) | 9496 (36.8) | 1884 (30.3) | 8923 (34.6) | ||
| Charlson comorbidity index | 3.5 ± 2.3 | 3.3 ± 2.3 | 0.068 | 3.4 ± 2.3 | 3.3 ± 2.3 | 0.020 |
| Atherosclerotic cardiovascular disease | ||||||
| Prior or current myocardial infarction | 2588 (42.0) | 10 872 (42.1) | 0.003 | 2595 (41.7) | 10 840 (42.1) | 0.006 |
| Prior coronary bypass graft surgery | 103 (1.7) | 336 (1.3) | 0.030 | 101 (1.6) | 362 (1.4) | 0.018 |
| Prior ischaemic stroke | 969 (15.7) | 4488 (17.4) | 0.045 | 1108 (17.8) | 4413 (17.1) | 0.019 |
| Peripheral artery disease | 399 (6.5) | 1349 (5.2) | 0.053 | 350 (5.6) | 1414 (5.5) | 0.007 |
| Medication before PCI | ||||||
| Aspirin | 4675 (75.8) | 15 932 (61.8) | 0.308 | 4288 (69.0) | 16 669 (64.7) | 0.092 |
| Clopidogrel | 3808 (61.8) | 12 005 (46.5) | 0.310 | 3172 (51.0) | 12 767 (49.5) | 0.030 |
| β-Blocker | 3222 (52.3) | 11 384 (44.1) | 0.164 | 3023 (48.6) | 11 827 (45.9) | 0.055 |
| RAAS blockade | 2966 (48.1) | 10 544 (40.9) | 0.146 | 2744 (44.2) | 10 930 (42.4) | 0.035 |
| Procedural information | ||||||
| Number of stents | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.023 | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.043 |
| Type of drug-eluting stent | ||||||
| First generationc | 1161 (18.8) | 3120 (12.1) | 0.187 | 781 (12.6) | 3435 (13.3) | 0.022 |
| Next generation | 5003 (81.2) | 22 682 (87.9) | 5434 (87.4) | 22 343 (86.7) | ||
| Duration of dual antiplatelet therapy | ||||||
| <365 days | 2610 (42.3) | 12 004 (46.5) | 0.084 | 2957 (47.6) | 11 836 (45.9) | 0.033 |
| ≥365 days | 3554 (57.7) | 13 798 (53.5) | 3258 (52.4) | 13 942 (54.1) | ||
| Year of PCI | ||||||
| 2005–2009 | 1370 (22.2) | 3878 (15.0) | 0.251 | 991 (16.0) | 4226 (16.4) | 0.066 |
| 2010–2014 | 1799 (29.2) | 6322 (24.5) | 1401 (22.5) | 6667 (25.1) | ||
| 2015–2016 | 2995 (48.6) | 15 602 (60.5) | 3823 (51.5) | 15 085 (58.5) | ||
| . | Before stabilized IPTW (N = 31 966) . | After stabilized IPTW (N = 31 993) . | ||||
|---|---|---|---|---|---|---|
| Characteristics . | Combination lipid-lowering therapy (N = 6164) . | High-intensity atorvastatin monotherapy (N = 25 802) . | SMD . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | SMD . |
| Age, years | 61.9 ± 10.9 | 62.9 ± 11.3 | 0.087 | 62.8 ± 11.0 | 62.7 ± 11.2 | 0.007 |
| Female | 1709 (27.7) | 6229 (24.1) | 0.082 | 1558 (25.1) | 6396 (24.8) | 0.006 |
| Comorbidity | ||||||
| Hypertension | 4730 (76.7) | 18 509 (71.7) | 0.115 | 4508 (72.5) | 18 717 (72.6) | 0.002 |
| Chronic kidney disease with severe renal impairmenta | 401 (6.5) | 1427 (5.5) | 0.041 | 392 (6.3) | 1485 (5.8) | 0.023 |
| Diabetes mellitus | 3033 (49.2) | 11 273 (43.7) | 0.111 | 2845 (45.8) | 11 534 (44.7) | 0.021 |
| Statin therapy before atorvastatin treatmentb | ||||||
| High-intensity statin | 1285 (20.8) | 11 098 (43.0) | 0.784 | 2664 (42.9) | 10 069 (39.1) | 0.097 |
| Low- to moderate-intensity statin | 3400 (55.2) | 5208 (20.2) | 1667 (26.8) | 6786 (26.3) | ||
| None | 1479 (24.0) | 9496 (36.8) | 1884 (30.3) | 8923 (34.6) | ||
| Charlson comorbidity index | 3.5 ± 2.3 | 3.3 ± 2.3 | 0.068 | 3.4 ± 2.3 | 3.3 ± 2.3 | 0.020 |
| Atherosclerotic cardiovascular disease | ||||||
| Prior or current myocardial infarction | 2588 (42.0) | 10 872 (42.1) | 0.003 | 2595 (41.7) | 10 840 (42.1) | 0.006 |
| Prior coronary bypass graft surgery | 103 (1.7) | 336 (1.3) | 0.030 | 101 (1.6) | 362 (1.4) | 0.018 |
| Prior ischaemic stroke | 969 (15.7) | 4488 (17.4) | 0.045 | 1108 (17.8) | 4413 (17.1) | 0.019 |
| Peripheral artery disease | 399 (6.5) | 1349 (5.2) | 0.053 | 350 (5.6) | 1414 (5.5) | 0.007 |
| Medication before PCI | ||||||
| Aspirin | 4675 (75.8) | 15 932 (61.8) | 0.308 | 4288 (69.0) | 16 669 (64.7) | 0.092 |
| Clopidogrel | 3808 (61.8) | 12 005 (46.5) | 0.310 | 3172 (51.0) | 12 767 (49.5) | 0.030 |
| β-Blocker | 3222 (52.3) | 11 384 (44.1) | 0.164 | 3023 (48.6) | 11 827 (45.9) | 0.055 |
| RAAS blockade | 2966 (48.1) | 10 544 (40.9) | 0.146 | 2744 (44.2) | 10 930 (42.4) | 0.035 |
| Procedural information | ||||||
| Number of stents | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.023 | 1.3 ± 0.6 | 1.3 ± 0.6 | 0.043 |
| Type of drug-eluting stent | ||||||
| First generationc | 1161 (18.8) | 3120 (12.1) | 0.187 | 781 (12.6) | 3435 (13.3) | 0.022 |
| Next generation | 5003 (81.2) | 22 682 (87.9) | 5434 (87.4) | 22 343 (86.7) | ||
| Duration of dual antiplatelet therapy | ||||||
| <365 days | 2610 (42.3) | 12 004 (46.5) | 0.084 | 2957 (47.6) | 11 836 (45.9) | 0.033 |
| ≥365 days | 3554 (57.7) | 13 798 (53.5) | 3258 (52.4) | 13 942 (54.1) | ||
| Year of PCI | ||||||
| 2005–2009 | 1370 (22.2) | 3878 (15.0) | 0.251 | 991 (16.0) | 4226 (16.4) | 0.066 |
| 2010–2014 | 1799 (29.2) | 6322 (24.5) | 1401 (22.5) | 6667 (25.1) | ||
| 2015–2016 | 2995 (48.6) | 15 602 (60.5) | 3823 (51.5) | 15 085 (58.5) | ||
Values are presented as the mean ± standard deviation or n (%).
IPTW, inverse probability of treatment weighting; SMD, standardized mean difference; PCI, percutaneous coronary intervention; RAAS, renin–angiotensin–aldosterone system.
Chronic kidney disease with advanced stage requiring intensive medical therapy and financial assistance from health insurance.
The intensity of prior statin treatment was categorized based on the 2013 American College of Cardiology/American Heart Association guideline for blood cholesterol treatment.
First-generation drug-eluting stent indicates Cypher and Taxus.
Risks of primary and secondary efficacy endpoints for 3 years in groups formed with stabilized inverse probability of treatment weighting
| . | . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| Composite of cardiovascular death, myocardial infarction, coronary artery revascularization, hospitalization for heart failure, and stroke | 1 year | 444 (7.2) | 2197 (8.5) | −1.3 (−2.1 to −0.6) | 0.80 (0.70−0.89) | <0.001 |
| 2 years | 701 (11.3) | 3272 (12.7) | −1.4 (−2.4 to −0.4) | 0.83 (0.74−0.88) | <0.001 | |
| 3 years | 803 (12.9) | 3901 (15.1) | −2.2 (−3.2 to −1.2) | 0.81 (0.74−0.88) | <0.001 | |
| Individual clinical endpoint | ||||||
| Cardiovascular death | 1 year | 78 (1.3) | 398 (1.5) | −0.2 (−0.6 to 0.1) | 0.80 (0.61−1.05) | 0.104 |
| 2 years | 142 (2.3) | 745 (2.9) | −0.6 (−1.1 to −0.1) | 0.79 (0.65−0.96) | 0.017 | |
| 3 years | 184 (3.0) | 1000 (3.9) | −0.9 (−1.4 to −0.4) | 0.77 (0.65−0.91) | 0.003 | |
| All-cause death | 1 year | 139 (2.2) | 596 (2.3) | −0.1 (−0.5 to 0.3) | 0.91 (0.74−1.12) | 0.364 |
| 2 years | 255 (4.1) | 1151 (4.5) | −0.4 (−0.9 to 0.2) | 0.89 (0.77−1.02) | 0.157 | |
| 3 years | 336 (5.4) | 1605 (6.2) | −0.8 (−1.5 to −0.1) | 0.85 (0.75−0.97) | 0.016 | |
| Myocardial infarction | 1 year | 80 (1.3) | 407 (1.6) | −0.3 (−0.6 to 0.0) | 0.76 (0.58−1.01) | 0.058 |
| 2 years | 89 (1.4) | 478 (1.9) | −0.4 (−0.7 to −0.1) | 0.75 (0.59−0.95) | 0.018 | |
| 3 years | 98 (1.6) | 529 (2.1) | −0.5 (−0.9 to −0.1) | 0.71 (0.56−0.91) | 0.005 | |
| Coronary artery revascularization | 1 year | 122 (2.0) | 490 (1.9) | 0.1 (−0.3 to 0.4) | 0.98 (0.79−1.21) | 0.857 |
| 2 years | 183 (2.9) | 869 (3.4) | −0.4 (−0.9 to 0.1) | 0.93 (0.79−1.10) | 0.407 | |
| 3 years | 214 (3.4) | 1042 (4.0) | −0.6 (−1.1 to −0.1) | 0.80 (0.69−0.94) | 0.006 | |
| Hospitalization for heart failure | 1 year | 150 (2.4) | 649 (2.5) | −0.1 (−0.5 to 0.3) | 0.92 (0.75−1.11) | 0.383 |
| 2 years | 237 (3.8) | 1057 (4.1) | −0.3 (−0.8 to −0.3) | 0.89 (0.76−1.04) | 0.139 | |
| 3 years | 305 (4.9) | 1314 (5.1) | −0.2 (−0.8 to 0.4) | 0.89 (0.78−1.02) | 0.117 | |
| Non-fatal stroke | 1 year | 86 (1.4) | 518 (2.0) | −0.6 (−1.0 to −0.2) | 0.86 (0.76−1.00) | 0.058 |
| 2 years | 131 (2.1) | 705 (2.7) | −0.6 (−1.1 to −0.2) | 0.77 (0.63−0.94) | 0.009 | |
| 3 years | 158 (2.5) | 853 (3.3) | −0.8 (−1.3 to −0.3) | 0.77 (0.65−0.93) | 0.006 | |
| . | . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| Composite of cardiovascular death, myocardial infarction, coronary artery revascularization, hospitalization for heart failure, and stroke | 1 year | 444 (7.2) | 2197 (8.5) | −1.3 (−2.1 to −0.6) | 0.80 (0.70−0.89) | <0.001 |
| 2 years | 701 (11.3) | 3272 (12.7) | −1.4 (−2.4 to −0.4) | 0.83 (0.74−0.88) | <0.001 | |
| 3 years | 803 (12.9) | 3901 (15.1) | −2.2 (−3.2 to −1.2) | 0.81 (0.74−0.88) | <0.001 | |
| Individual clinical endpoint | ||||||
| Cardiovascular death | 1 year | 78 (1.3) | 398 (1.5) | −0.2 (−0.6 to 0.1) | 0.80 (0.61−1.05) | 0.104 |
| 2 years | 142 (2.3) | 745 (2.9) | −0.6 (−1.1 to −0.1) | 0.79 (0.65−0.96) | 0.017 | |
| 3 years | 184 (3.0) | 1000 (3.9) | −0.9 (−1.4 to −0.4) | 0.77 (0.65−0.91) | 0.003 | |
| All-cause death | 1 year | 139 (2.2) | 596 (2.3) | −0.1 (−0.5 to 0.3) | 0.91 (0.74−1.12) | 0.364 |
| 2 years | 255 (4.1) | 1151 (4.5) | −0.4 (−0.9 to 0.2) | 0.89 (0.77−1.02) | 0.157 | |
| 3 years | 336 (5.4) | 1605 (6.2) | −0.8 (−1.5 to −0.1) | 0.85 (0.75−0.97) | 0.016 | |
| Myocardial infarction | 1 year | 80 (1.3) | 407 (1.6) | −0.3 (−0.6 to 0.0) | 0.76 (0.58−1.01) | 0.058 |
| 2 years | 89 (1.4) | 478 (1.9) | −0.4 (−0.7 to −0.1) | 0.75 (0.59−0.95) | 0.018 | |
| 3 years | 98 (1.6) | 529 (2.1) | −0.5 (−0.9 to −0.1) | 0.71 (0.56−0.91) | 0.005 | |
| Coronary artery revascularization | 1 year | 122 (2.0) | 490 (1.9) | 0.1 (−0.3 to 0.4) | 0.98 (0.79−1.21) | 0.857 |
| 2 years | 183 (2.9) | 869 (3.4) | −0.4 (−0.9 to 0.1) | 0.93 (0.79−1.10) | 0.407 | |
| 3 years | 214 (3.4) | 1042 (4.0) | −0.6 (−1.1 to −0.1) | 0.80 (0.69−0.94) | 0.006 | |
| Hospitalization for heart failure | 1 year | 150 (2.4) | 649 (2.5) | −0.1 (−0.5 to 0.3) | 0.92 (0.75−1.11) | 0.383 |
| 2 years | 237 (3.8) | 1057 (4.1) | −0.3 (−0.8 to −0.3) | 0.89 (0.76−1.04) | 0.139 | |
| 3 years | 305 (4.9) | 1314 (5.1) | −0.2 (−0.8 to 0.4) | 0.89 (0.78−1.02) | 0.117 | |
| Non-fatal stroke | 1 year | 86 (1.4) | 518 (2.0) | −0.6 (−1.0 to −0.2) | 0.86 (0.76−1.00) | 0.058 |
| 2 years | 131 (2.1) | 705 (2.7) | −0.6 (−1.1 to −0.2) | 0.77 (0.63−0.94) | 0.009 | |
| 3 years | 158 (2.5) | 853 (3.3) | −0.8 (−1.3 to −0.3) | 0.77 (0.65−0.93) | 0.006 | |
A 95% confidence interval (CI) for absolute risk reduction attributed to each treatment was calculated.
Cox proportional hazard models were used to obtain adjusted hazard ratio, 95% CI, and P-values by defining high-intensity atorvastatin monotherapy as the reference arm.
Risks of primary and secondary efficacy endpoints for 3 years in groups formed with stabilized inverse probability of treatment weighting
| . | . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| Composite of cardiovascular death, myocardial infarction, coronary artery revascularization, hospitalization for heart failure, and stroke | 1 year | 444 (7.2) | 2197 (8.5) | −1.3 (−2.1 to −0.6) | 0.80 (0.70−0.89) | <0.001 |
| 2 years | 701 (11.3) | 3272 (12.7) | −1.4 (−2.4 to −0.4) | 0.83 (0.74−0.88) | <0.001 | |
| 3 years | 803 (12.9) | 3901 (15.1) | −2.2 (−3.2 to −1.2) | 0.81 (0.74−0.88) | <0.001 | |
| Individual clinical endpoint | ||||||
| Cardiovascular death | 1 year | 78 (1.3) | 398 (1.5) | −0.2 (−0.6 to 0.1) | 0.80 (0.61−1.05) | 0.104 |
| 2 years | 142 (2.3) | 745 (2.9) | −0.6 (−1.1 to −0.1) | 0.79 (0.65−0.96) | 0.017 | |
| 3 years | 184 (3.0) | 1000 (3.9) | −0.9 (−1.4 to −0.4) | 0.77 (0.65−0.91) | 0.003 | |
| All-cause death | 1 year | 139 (2.2) | 596 (2.3) | −0.1 (−0.5 to 0.3) | 0.91 (0.74−1.12) | 0.364 |
| 2 years | 255 (4.1) | 1151 (4.5) | −0.4 (−0.9 to 0.2) | 0.89 (0.77−1.02) | 0.157 | |
| 3 years | 336 (5.4) | 1605 (6.2) | −0.8 (−1.5 to −0.1) | 0.85 (0.75−0.97) | 0.016 | |
| Myocardial infarction | 1 year | 80 (1.3) | 407 (1.6) | −0.3 (−0.6 to 0.0) | 0.76 (0.58−1.01) | 0.058 |
| 2 years | 89 (1.4) | 478 (1.9) | −0.4 (−0.7 to −0.1) | 0.75 (0.59−0.95) | 0.018 | |
| 3 years | 98 (1.6) | 529 (2.1) | −0.5 (−0.9 to −0.1) | 0.71 (0.56−0.91) | 0.005 | |
| Coronary artery revascularization | 1 year | 122 (2.0) | 490 (1.9) | 0.1 (−0.3 to 0.4) | 0.98 (0.79−1.21) | 0.857 |
| 2 years | 183 (2.9) | 869 (3.4) | −0.4 (−0.9 to 0.1) | 0.93 (0.79−1.10) | 0.407 | |
| 3 years | 214 (3.4) | 1042 (4.0) | −0.6 (−1.1 to −0.1) | 0.80 (0.69−0.94) | 0.006 | |
| Hospitalization for heart failure | 1 year | 150 (2.4) | 649 (2.5) | −0.1 (−0.5 to 0.3) | 0.92 (0.75−1.11) | 0.383 |
| 2 years | 237 (3.8) | 1057 (4.1) | −0.3 (−0.8 to −0.3) | 0.89 (0.76−1.04) | 0.139 | |
| 3 years | 305 (4.9) | 1314 (5.1) | −0.2 (−0.8 to 0.4) | 0.89 (0.78−1.02) | 0.117 | |
| Non-fatal stroke | 1 year | 86 (1.4) | 518 (2.0) | −0.6 (−1.0 to −0.2) | 0.86 (0.76−1.00) | 0.058 |
| 2 years | 131 (2.1) | 705 (2.7) | −0.6 (−1.1 to −0.2) | 0.77 (0.63−0.94) | 0.009 | |
| 3 years | 158 (2.5) | 853 (3.3) | −0.8 (−1.3 to −0.3) | 0.77 (0.65−0.93) | 0.006 | |
| . | . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| Composite of cardiovascular death, myocardial infarction, coronary artery revascularization, hospitalization for heart failure, and stroke | 1 year | 444 (7.2) | 2197 (8.5) | −1.3 (−2.1 to −0.6) | 0.80 (0.70−0.89) | <0.001 |
| 2 years | 701 (11.3) | 3272 (12.7) | −1.4 (−2.4 to −0.4) | 0.83 (0.74−0.88) | <0.001 | |
| 3 years | 803 (12.9) | 3901 (15.1) | −2.2 (−3.2 to −1.2) | 0.81 (0.74−0.88) | <0.001 | |
| Individual clinical endpoint | ||||||
| Cardiovascular death | 1 year | 78 (1.3) | 398 (1.5) | −0.2 (−0.6 to 0.1) | 0.80 (0.61−1.05) | 0.104 |
| 2 years | 142 (2.3) | 745 (2.9) | −0.6 (−1.1 to −0.1) | 0.79 (0.65−0.96) | 0.017 | |
| 3 years | 184 (3.0) | 1000 (3.9) | −0.9 (−1.4 to −0.4) | 0.77 (0.65−0.91) | 0.003 | |
| All-cause death | 1 year | 139 (2.2) | 596 (2.3) | −0.1 (−0.5 to 0.3) | 0.91 (0.74−1.12) | 0.364 |
| 2 years | 255 (4.1) | 1151 (4.5) | −0.4 (−0.9 to 0.2) | 0.89 (0.77−1.02) | 0.157 | |
| 3 years | 336 (5.4) | 1605 (6.2) | −0.8 (−1.5 to −0.1) | 0.85 (0.75−0.97) | 0.016 | |
| Myocardial infarction | 1 year | 80 (1.3) | 407 (1.6) | −0.3 (−0.6 to 0.0) | 0.76 (0.58−1.01) | 0.058 |
| 2 years | 89 (1.4) | 478 (1.9) | −0.4 (−0.7 to −0.1) | 0.75 (0.59−0.95) | 0.018 | |
| 3 years | 98 (1.6) | 529 (2.1) | −0.5 (−0.9 to −0.1) | 0.71 (0.56−0.91) | 0.005 | |
| Coronary artery revascularization | 1 year | 122 (2.0) | 490 (1.9) | 0.1 (−0.3 to 0.4) | 0.98 (0.79−1.21) | 0.857 |
| 2 years | 183 (2.9) | 869 (3.4) | −0.4 (−0.9 to 0.1) | 0.93 (0.79−1.10) | 0.407 | |
| 3 years | 214 (3.4) | 1042 (4.0) | −0.6 (−1.1 to −0.1) | 0.80 (0.69−0.94) | 0.006 | |
| Hospitalization for heart failure | 1 year | 150 (2.4) | 649 (2.5) | −0.1 (−0.5 to 0.3) | 0.92 (0.75−1.11) | 0.383 |
| 2 years | 237 (3.8) | 1057 (4.1) | −0.3 (−0.8 to −0.3) | 0.89 (0.76−1.04) | 0.139 | |
| 3 years | 305 (4.9) | 1314 (5.1) | −0.2 (−0.8 to 0.4) | 0.89 (0.78−1.02) | 0.117 | |
| Non-fatal stroke | 1 year | 86 (1.4) | 518 (2.0) | −0.6 (−1.0 to −0.2) | 0.86 (0.76−1.00) | 0.058 |
| 2 years | 131 (2.1) | 705 (2.7) | −0.6 (−1.1 to −0.2) | 0.77 (0.63−0.94) | 0.009 | |
| 3 years | 158 (2.5) | 853 (3.3) | −0.8 (−1.3 to −0.3) | 0.77 (0.65−0.93) | 0.006 | |
A 95% confidence interval (CI) for absolute risk reduction attributed to each treatment was calculated.
Cox proportional hazard models were used to obtain adjusted hazard ratio, 95% CI, and P-values by defining high-intensity atorvastatin monotherapy as the reference arm.
| . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|
| Discontinuation of statin | 519 (8.4) | 2571 (10.0) | −1.6 (−2.5 to −0.8) | 0.81 (0.73−0.90) | <0.001 |
| New-onset diabetes requiring medicationc | 237/3370 (7.0) | 1253/14 244 (8.8) | −1.7 (−2.9 to −0.6) | 0.80 (0.70−0.92) | 0.002 |
| Rhabdomyolysis | 14 (0.2) | 90 (0.3) | −0.1 (−0.3 to 0.1) | 0.80 (0.47−1.34) | 0.390 |
| Cholecystectomy | 71 (1.1) | 236 (0.9) | 0.2 (0.0 to 0.5) | 1.27 (0.96−1.70) | 0.098 |
| Cancer diagnosis | 246 (4.0) | 1037 (4.0) | 0.0 (−0.6 to 0.5) | 0.98 (0.84−1.14) | 0.799 |
| . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|
| Discontinuation of statin | 519 (8.4) | 2571 (10.0) | −1.6 (−2.5 to −0.8) | 0.81 (0.73−0.90) | <0.001 |
| New-onset diabetes requiring medicationc | 237/3370 (7.0) | 1253/14 244 (8.8) | −1.7 (−2.9 to −0.6) | 0.80 (0.70−0.92) | 0.002 |
| Rhabdomyolysis | 14 (0.2) | 90 (0.3) | −0.1 (−0.3 to 0.1) | 0.80 (0.47−1.34) | 0.390 |
| Cholecystectomy | 71 (1.1) | 236 (0.9) | 0.2 (0.0 to 0.5) | 1.27 (0.96−1.70) | 0.098 |
| Cancer diagnosis | 246 (4.0) | 1037 (4.0) | 0.0 (−0.6 to 0.5) | 0.98 (0.84−1.14) | 0.799 |
A 95% confidence interval (CI) for absolute risk reduction attributed to each treatment was calculated.
Cox proportional hazard models were used to obtain adjusted hazard ratio, 95% CI, and P-values by defining high-intensity atorvastatin monotherapy as the reference arm.
Incidence of new-onset diabetes requiring medication was assessed for participants without prior diabetes history at enrolment.
| . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|
| Discontinuation of statin | 519 (8.4) | 2571 (10.0) | −1.6 (−2.5 to −0.8) | 0.81 (0.73−0.90) | <0.001 |
| New-onset diabetes requiring medicationc | 237/3370 (7.0) | 1253/14 244 (8.8) | −1.7 (−2.9 to −0.6) | 0.80 (0.70−0.92) | 0.002 |
| Rhabdomyolysis | 14 (0.2) | 90 (0.3) | −0.1 (−0.3 to 0.1) | 0.80 (0.47−1.34) | 0.390 |
| Cholecystectomy | 71 (1.1) | 236 (0.9) | 0.2 (0.0 to 0.5) | 1.27 (0.96−1.70) | 0.098 |
| Cancer diagnosis | 246 (4.0) | 1037 (4.0) | 0.0 (−0.6 to 0.5) | 0.98 (0.84−1.14) | 0.799 |
| . | Combination lipid-lowering therapy (N = 6215) . | High-intensity atorvastatin monotherapy (N = 25 778) . | Risk difference (95% CI)a . | Hazard ratio (95% CI)b . | P-value . |
|---|---|---|---|---|---|
| Discontinuation of statin | 519 (8.4) | 2571 (10.0) | −1.6 (−2.5 to −0.8) | 0.81 (0.73−0.90) | <0.001 |
| New-onset diabetes requiring medicationc | 237/3370 (7.0) | 1253/14 244 (8.8) | −1.7 (−2.9 to −0.6) | 0.80 (0.70−0.92) | 0.002 |
| Rhabdomyolysis | 14 (0.2) | 90 (0.3) | −0.1 (−0.3 to 0.1) | 0.80 (0.47−1.34) | 0.390 |
| Cholecystectomy | 71 (1.1) | 236 (0.9) | 0.2 (0.0 to 0.5) | 1.27 (0.96−1.70) | 0.098 |
| Cancer diagnosis | 246 (4.0) | 1037 (4.0) | 0.0 (−0.6 to 0.5) | 0.98 (0.84−1.14) | 0.799 |
A 95% confidence interval (CI) for absolute risk reduction attributed to each treatment was calculated.
Cox proportional hazard models were used to obtain adjusted hazard ratio, 95% CI, and P-values by defining high-intensity atorvastatin monotherapy as the reference arm.
Incidence of new-onset diabetes requiring medication was assessed for participants without prior diabetes history at enrolment.
Discussion
Taking advantage of the Korean NHIS database system, which can accurately track medication prescription data at the drug dosage level, we investigated whether the benefits of combination therapy with ezetimibe and moderate-intensity rosuvastatin, as explored in the RACING trial, could be extrapolated to atorvastatin therapy. The principal findings of the present study are as follows. (1) For secondary prevention in patients with DES implantation, the combination of moderate-intensity atorvastatin and ezetimibe was significantly associated with more favourable clinical outcomes than high-intensity atorvastatin monotherapy. (2) Combination lipid-lowering therapy was also associated with better drug adherence and a lower risk of new-onset diabetes requiring medication than high-intensity atorvastatin monotherapy. (3) These findings concur with the results from the randomized RACING trial, which examined the effects of rosuvastatin use.
Despite notable progress in procedural methods, the enhanced characteristics of contemporary DESs, and advancements in medical therapies, patients who undergo PCI continue to confront a significant long-term risk of adverse cardiovascular events.12 A recent report from the National Cardiovascular Data Registry's CathPCI Registry, which contains 723 644 cases of PCI in patients aged 65 and above, revealed that one in six patients encountered adverse cardiovascular events within 1-year of the index procedure.13 In this context, current lipid management guidelines emphasize strict control of LDL-C levels in patients with ASCVD, and subgroups with previous acute coronary syndrome or a history of PCI are considered to be at particularly high risk.14,15 The assertions suggested by the dyslipidaemia guidelines are reinforced by empirical evidence from real-world practice.2,16 In a Canadian cohort study of patients who underwent DES implantation (n = 47 884), patients with post-procedure LDL-C >100 mg/dL had a significantly higher risk of adverse cardiovascular events than those with LDL-C <70 mg/dL.2 Similarly, a Swedish cohort of 40 607 patients hospitalized with MI revealed that achieving ≥50% LDL-C reduction via high-intensity statins at discharge produced a lower incidence of composite cardiovascular events over a median follow-up period 3.8 years than was found in those prescribed lower-intensity statins.16 Nevertheless, despite the cumulative evidence from research and subsequent guideline endorsements promoting the use of high-intensity statins for adequate LDL-C reduction in patients with coronary artery disease, a pronounced discrepancy has persisted between the recommendations and actual clinical practice.4,5,17
In a large contemporary cohort of patients with coronary artery disease from the USA (n = 416 349), the prescription rate of a high-intensity statin was only 22.5%, and more alarmingly, 49.9% of patients with a history of ASCVD did not receive any statin therapy.18 Additionally, in Swedish national registry data that included 192 435 patients with very high risk ASCVD who began taking a moderate-intensity statin instead of a high-intensity statin, no up-titration to a high-intensity statin was observed in 72% of patients, even though early up-titration to a high-intensity statin was significantly associated with favourable clinical outcomes.19 These collective findings underscore a significant disparity between evidence-based knowledge and real-world clinical practice, indicating a notable reluctance among physicians to initiate or intensify statin therapy to a high-intensity level, plausibly due to concerns about drug-related side effects such as statin-associated muscle symptoms or the occurrence of diabetes mellitus.20–22
Recent results from the randomized RACING trial demonstrated that combining moderate-intensity rosuvastatin with ezetimibe had therapeutic efficacy comparable to that of high-intensity rosuvastatin monotherapy in terms of 3-year composite cardiovascular events, and the combination therapy was also associated with more favourable LDL-C reduction and drug adherence among ASCVD patients who had undergone PCI.6,23 In addition, real-world data from a Korean nationwide claims data cohort showed that combination therapy with moderate-intensity rosuvastatin plus ezetimibe was superior to high-intensity rosuvastatin monotherapy in terms of adverse cardiovascular events in patients undergoing PCI, with a significantly lower incidence of drug-related adverse events such as statin discontinuation and new-onset events requiring medication.10 Along with rosuvastatin, atorvastatin is the representative high-intensity statin most commonly prescribed for patients with ASCVD because it has accumulated the largest amount of favourable evidence.24,25 There are controversial data regarding the comparative therapeutic impact of atorvastatin vs. rosuvastatin with respect to efficacy and safety. In a prospective cohort of 38 023 patients with acute coronary syndrome, which investigated the clinical outcomes of upfront combination lipid-lowering therapy, mortality was significantly lower in those treated with rosuvastatin vs. atorvastatin (odds ratio [OR] 0.79, 95% CI 0.72–0.85).26 Meanwhile, in a large-volume cohort meta-analysis of 4 143 517 patients on statin therapy, the prevalence of statin intolerance was comparable between the atorvastatin and rosuvastatin.27 Furthermore, the actual clinical benefit of combination therapy with atorvastatin and ezetimibe for patients with DES implantation has not yet been sufficiently established. This study revealed the beneficial effects of combining moderate-intensity atorvastatin and ezetimibe in a large cohort of patients in daily practice.
In a recent study of 1499 patients with acute MI, lipid-lowering therapy was de-escalated in 24% and intensified in 9% of this very high risk population at 12 months after discharge.28 The reduction of LDL-C >50% was achieved in 29% of patients on high-intensity statin therapy and 42% on a combination of high-intensity statin with ezetimibe. In addition, in patients with acute coronary syndrome, upfront combination lipid-lowering therapy was associated with significantly lower mortality compared with statin monotherapy (OR 0.53, 95% CI 0.38–0.73).26 The latest European guidelines for acute coronary syndrome support the combination lipid-lowering therapy of ezetimibe with statin as a class IIb recommendation.29 Taken together, for patients at high risk of ASCVD, upfront combination lipid-lowering therapy with ezetimibe plus high- or moderate-intensity statin could be considered as a primary treatment strategy.
Limitations
This study has several limitations. First, we did not analyse serial changes in LDL-C and other lipid profiles during the follow-up period because the claims data do not contain that information. Second, because the NHIS database contains only claims data and not medical records, we could not discern the reasons for statin discontinuation or the occurrence of statin-associated muscle symptoms not diagnosed as rhabdomyolysis. Third, due to the inherent limitations of the retrospective cohort study based on claims data, such as potential reverse causality, data drop-out, and missing data regarding concomitant therapies, the findings of this investigation cannot ascertain causal associations, and residual confounding factors might remain despite the application of stabilized IPTW.10 Future prospective randomized trials are needed to confirm these findings.
Conclusion
In this nationwide cohort study, the combination of moderate-intensity atorvastatin and ezetimibe was associated with a lower occurrence of adverse cardiovascular events, statin discontinuation, and the emergence of new-onset diabetes requiring medication than high-intensity atorvastatin monotherapy in patients who had undergone PCI with DES implantation. The current real-world data support the suitability of upfront combination lipid-lowering therapy as a primary treatment strategy in patients at high risk of ASCVD.
Acknowledgments
The funder had no role in the design and conduct of the study; data collection, management, statistical analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The study complies with the Declaration of Helsinki, and the study was approved by the institutional review boards and independent ethics committees for all participating centres. All participants provided written informed consent.
Funding
Cardiovascular Research Center, Seoul, Korea.
Conflict of interest: No authors have any interests to disclose.
Data availability
The datasets generated for the analyses are not publicly available because of strict government restrictions.
Author contributions
S.J.L., J.H.J., C.M.N., and M.K.H. contributed to the conception and design. S.J.L. and M.K.H. wrote the study protocol. J.H.J. and C.M.N. performed the programming to extract data from the NHIS database. S.J.L., J.H.J., C.M.N., and M.K.H. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S.J.L., J.H.J., C.N.M., and M.H.K. verified the data and conducted all analyses. All authors provided a critical review of the manuscript. All authors read and approved the final publication. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
References
Author notes
These authors contributed equally to this work.
- atherosclerosis
- atorvastatin
- dyslipidemias
- myocardial infarction
- percutaneous coronary intervention
- coronary arteriosclerosis
- coronary artery
- statins
- cardiovascular diseases
- diabetes mellitus
- ischemic stroke
- heart failure
- combined modality therapy
- safety
- lipids
- treatment outcome
- lipid-lowering therapy
- ezetimibe
- drug-eluting stents
- revascularization
- rosuvastatin
- medication adherence
- cardiovascular death
- atorvastatin/ezetimibe



