-
PDF
- Split View
-
Views
-
Cite
Cite
Mariusz Kruk, Cezary Kępka, The unified atherosclerosis paradigm: promisingly close or perpetually distant?, European Heart Journal - Cardiovascular Imaging, Volume 25, Issue 2, February 2024, Pages 173–174, https://doi.org/10.1093/ehjci/jead254
Close - Share Icon Share
This editorial refers to ‘Distal-Vessel Fractional Flow Reserve by Computed Tomography to Monitor Epicardial Coronary Artery Disease’, by M. Chen et al., https://doi.org/10.1093/ehjci/jead229.
Despite extensive research and significant management advances, coronary atherosclerosis remains the most common cause of death globally. Its progression is non-uniform and hardly predictable in terms of the specific location and timing. The phenomenon is the result of interplay between systemic and local factors, embracing both coronary lumen and wall and may manifest from slow or accelerated build up of coronary plaque to acute disruptions with corresponding clinical sequelle.
The current diagnostic and management approach for coronary atherosclerosis has evolved due to constraints imposed by the available diagnostic tools, which lack the ability to non-invasively and routinely identify coronary atherosclerosis or its high-risk features in its natural context. This limitation has led to a development of seemingly separate and infrequently overlapping domains. The first primarily emphasizes coronary artery lumen and focuses on reparative considerations, including optimal qualifying criteria and methods for interventional stenosis management. Conversely, the second domain aligns more with the preventive medicine and the management of cardiovascular risks.1 The introduction of coronary computed tomography angiography (CCTA) into clinical practice marks a significant shift from the previous paradigm. CCTA not only offers extensive data on both the coronary lumen and coronary wall composition but also comprise a pre-requisite for simulations modelling physiological processes, such as fractional flow reserve derived from CCTA (FFR-CT). Ongoing advancements encompass the evaluation of Fat Attenuation Index (FAI), simulation of plaque biomechanics, or radiomic plaque analysis, supported by exploding applications of artificial intelligence2,3 (Figure 1). Our evolving comprehension of atherosclerosis and its early stages has begun to significantly influence our approach to both understanding and treating the condition. The continuous influx of data and improved insights into the evolution of vulnerable plaques has paved the way for a potential paradigm shift in how we classify atherosclerosis. This shift might mirror the classifications used in oncology, focusing more on plaque morphology and associated risks rather than relying solely on clinical symptoms or ‘lumenology’. This refined classification could be a game-changer, allowing us to tailor therapeutic strategies more precisely, even before visible clinical symptoms manifest. We would be able to intervene earlier, employing advanced methods such as more specific systemic or local mechanical treatments aimed at stabilizing vulnerable plaques, regardless of their immediate physiological impact, to potentially prevent acute coronary syndrome (ACS) from occurring.1
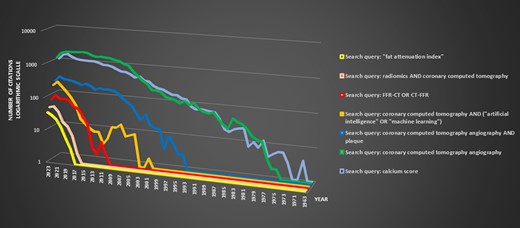
Dynamic representation and timeline of evolving concepts in coronary computed tomography: an analysis of PubMed research publications. The chart illustrates the annual publication count for specific Boolean search queries (legend provides search details) related to coronary computed tomography on the X-axis, while the Y-axis represents the citation count on a logarithmic scale. Based on the chart it may be inferred, that the dynamics of the newest AI-based developments within the area of coronary computed tomography angiography is exponential, giving hope for rapid accumulation of the knowledge and perhaps its faster clinical application.
In the current study, Chen et al. explored an intriguing concept assessing the progression of atherosclerosis using FFR-CT, which serves as a reflection of changes in epicardial atherosclerosis. The authors evaluated parameters that correlated with distal-vessel FFR-CT, and determined the combination of clinical and CCTA parameters correlated to its changes. The study found that improvement of distal-vessel FFR-CT was captured by a decrease in high-risk plaque and increases in lumen volume and remodelling index, whereas increases in stenosis diameter ratio, medium density calcified plaque volume, and total cholesterol presaged worsening of the FFR-CT. Authors conclude by asserting that the distal-vessel FFR-CT facilitates an encompassing evaluation of epicardial atherosclerotic plaque burden on a vessel-specific basis, offering a sequential means to discern alterations in global coronary artery disease (CAD).4
While atherosclerosis primarily concerns the coronary arterial wall, its paramount effects are witnessed within the lumen. This study underscores the cruciality of this fact. Primarily, it elucidates the capability of CCTA to amalgamate a vast array of anatomical and physiological data, augmenting our understanding of CAD and its progression, with the potential to improve patient outcomes. Nevertheless, this trajectory is anything but simple; rather, it introduces an additional level of complexity to the already intricate and underexplored realm of cardiovascular risk assessment, monitoring, and management. Presently, we lack comprehensive data to fully navigate this evolving multidimensional cardiovascular risk landscape and implement precise, evidence-based interventions, whether on a local or systemic scale. To achieve this, further data, inclusive of outcome studies, are imperative. If the initial phase effectively showcases the clinical usefulness of these innovative approaches, the next significant step is integrating these findings into routine clinical operations. However, this transition comes with its distinctive set of difficulties. It entails creating and validating specialized tools, establishing reimbursement structures, re-organizing workflows, and providing comprehensive personnel training. Ideally, this process should be coordinated by cardiac societies for optimal outcomes. It is notable that, although the initial publications highlighting the clinical value of FFR-CT—a clinically validated and seemingly straightforward method—were published over a decade ago, its widespread integration into clinical practice is still pending, and it is still not supported by European guidelines3,5 (Figure 1).
In conclusion, while CCTA holds the potential to comprehensively integrate the multidimensional framework of coronary atherosclerosis, we should expect that before achieving simplicity and clinical applicability, this process may become even more intricate.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of EHJCI, the European Heart Rhythm Association or the European Society of Cardiology.
Conflict of interest: None declared.



