-
PDF
- Split View
-
Views
-
Cite
Cite
J Bennett, G Thornton, C Nitsche, F Gama, R Mravljak, A Abiodun, N Aziminia, E Gautam-Aitken, I Pierce, P Kellman, C Manisty, A Hughes, R Davies, J C Moon, T A Treibel, Left ventricular cellular regression occurs without matrix changes at two months post aortic valve replacement in severe aortic stenosis, European Heart Journal - Cardiovascular Imaging, Volume 24, Issue Supplement_1, June 2023, jead119.123, https://doi.org/10.1093/ehjci/jead119.123
Close - Share Icon Share
Abstract
Type of funding sources: Foundation. Main funding source(s): British Heart Foundation
Increased left ventricular mass (LVM) in severe aortic stenosis (AS) is a result of the combined expansion of myocardial cellular and extracellular compartments in response to chronic pressure overload. Upon relief of pressure overload by aortic valve replacement (AVR), LVM regresses significantly however patients remain at risk of post-AVR heart failure and mortality.
T1 mapping, by cardiovascular magnetic resonance (CMR), can quantify the proportion of cellular and extracellular volume.
We sought to determine the relative contributions of cellular and extracellular remodelling early after AVR to gain insight into potential post-AVR mechanisms of heart failure.
Patients with severe symptomatic AS underwent CMR (volumes, function, pre- and post-contrast T1 mapping using 0.1mmol/kg Dotarem) prior to and 8-weeks after surgical or transcatheter AVR. Global extracellular volume fraction (ECV%) was quantified from pre- and post-contrast T1 mapping and synthetic haematocrit. ECV% was used to calculate cellular compartment volume (CellVol) and extracellular volume (ECVol) from total LV volume (LVM/1.05[specific gravity of myocardium]). Segments with focal LGE were excluded from ECV% calculation.
Statistical analysis was via paired t-test denoted by standard deviations (SD), or Wilcoxon signed-rank test with reported interquartile ranges (IQR).
27 patients (age 71.2 SD±9.8, male 70.3%) returned for repeat investigation at median 8.5 [IQR 7.1–11.6] weeks post-AVR (surgical: 16 / transcatheter: 11). Aortic valve peak velocity significantly improved (4.5 SD±0.5m/s to 2.4 SD±0.4m/s; p<0.001) alongside median NYHA class (2 [IQR 0] to 1 [IQR 0.5]; p<0.001).
LVM regressed 10.3% (170.8 SD±36.6g to 153.2 SD±39g; P<0.001), which comprised of a 13.8% reduction in CellVol (134.8 SD±29.4mL to 116.2 SD±30.5mL; P<0.001). However, ECVol remained unchanged (44.4 SD±10.2mL to 44.7 SD±11.2mL; P=0.78) resulting in an ECV% increase of 3.1% (24.8 SD±2.4% to 27.9 SD±2.1%; P<0.001).
We show for the first time that the rapid early LVM reduction after AVR is driven entirely by regression of cardiomyocyte hypertrophy. The extracellular compartment volume is unchanged at two months post-AVR. This relative increase in the ratio of fibrosis and cell volume may result in early worsening myocardial stiffness and could be a contributing factor to post-AVR diastolic dysfunction and heart failure.
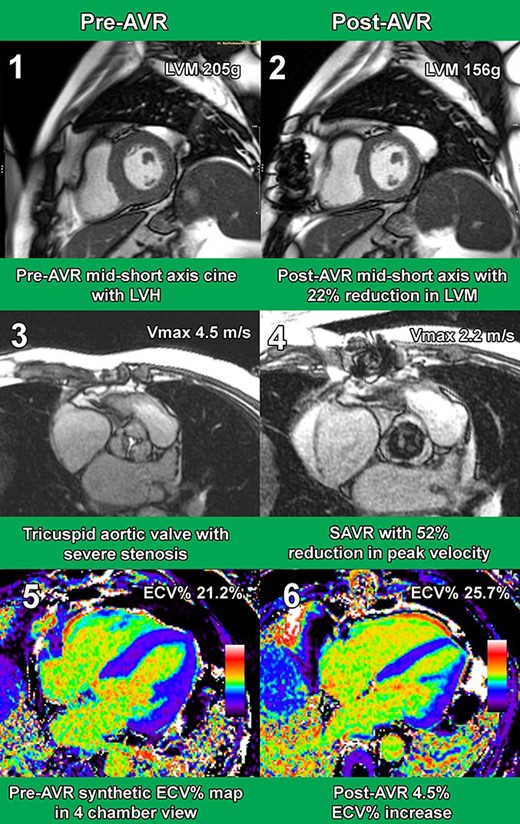
Exemplar Case CMR Images
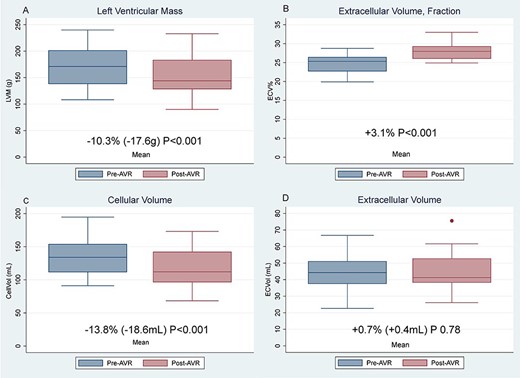
Graphs LVM, CellVol, ECVol, & ECV%
- aortic valve
- aortic valve stenosis
- cardiac myocytes
- myocardium
- heart failure, diastolic
- heart failure
- left ventricle
- aortic valve replacement
- cell volume
- fibrosis
- surgical procedures, operative
- heart
- hematocrit procedure
- hematocrit
- hypertrophy
- mortality
- specific gravity
- cardiac mri
- graphical displays
- paired t-test
- new york heart association classification
- total liquid ventilation
- linear gingival erythema
- megalencephalic leukoencephalopathy with subcortical cysts
- peak arterial velocity
- gadoterate meglumine



