-
PDF
- Split View
-
Views
-
Cite
Cite
Maxim J P Rooijakkers, Niels A Stens, Marleen H van Wely, Kees van der Wulp, Laura Rodwell, Helmut Gehlmann, Leen A F M van Garsse, Guillaume S C Geuzebroek, Michel W A Verkroost, Jesse Habets, Saloua El Messaoudi, Dick H J Thijssen, Robin Nijveldt, Niels van Royen, Diastolic delta best predicts paravalvular regurgitation after transcatheter aortic valve replacement as assessed by cardiac magnetic resonance: the APPOSE trial, European Heart Journal - Cardiovascular Imaging, Volume 24, Issue 8, August 2023, Pages 1072–1081, https://doi.org/10.1093/ehjci/jead033
Close - Share Icon Share
Abstract
Paravalvular regurgitation (PVR) is a common complication after transcatheter aortic valve replacement (TAVR) that poses an increased risk of rehospitalization for heart failure and mortality. The aim of this study was to assess the accuracy of haemodynamic indices to predict relevant PVR.
In this prospective single-centre clinical trial, four haemodynamic indices of PVR measured during TAVR were assessed for their correlation with gold standard cardiac magnetic resonance (CMR)-derived regurgitant fraction (CMR-RF) at 1 month follow-up: diastolic delta (DD), heart rate-adjusted diastolic delta (HR-DD), aortic regurgitation index (ARI), and aortic regurgitation index ratio (ARI ratio). These haemodynamic indices were analysed for their ability to predict relevant PVR (defined as CMR-RF > 20%) using receiver operating characteristic (ROC) curves with corresponding area under the ROC curves (AUCs). A total of 77 patients were included and had CMR performed 41 ± 14 days after TAVR. Mean CMR-RF was 12.4 ± 9.3%. Fifteen (19.5%) patients had CMR-RF > 20%. DD had the best correlation with CMR-RF and the highest AUC to predict relevant PVR (0.82; 95% CI, 0.72–0.92), followed by HR-DD (AUC 0.78; 95% CI, 0.67–0.89), ARI (AUC 0.78; 95% CI, 0.66–0.89), and ARI ratio (AUC 0.65; 95% CI, 0.49–0.81). The optimal cut-off value for DD was 32 mmHg, with sensitivity of 69% and specificity of 77% in predicting relevant PVR.
DD measured during TAVR best predicts relevant PVR. Correction for heart rate (HR-DD) or systolic blood pressure (ARI, ARI ratio) did not improve this predictive value.
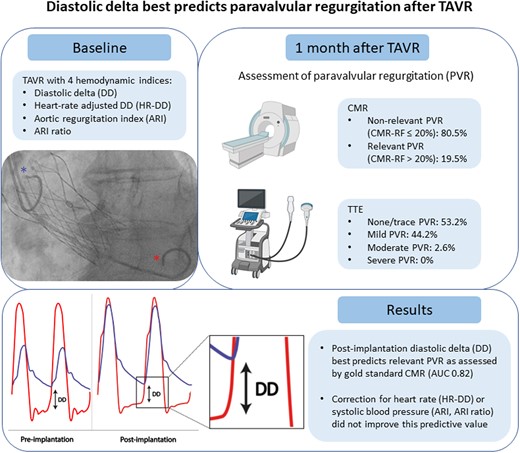
Schematic overview of the procedures and main results of the study. The blue and red asterisks denote pigtail catheters measuring pressure in the aorta and left ventricle, respectively. Haemodynamic indices were derived from these pressure measurements. AUC, area under the curve; CMR, cardiac magnetic resonance; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiography.
Introduction
Transcatheter aortic valve replacement (TAVR) is a well-established minimally invasive treatment option in patients with severe symptomatic aortic stenosis (AS). Initially established for inoperable patients at high or prohibitive surgical risk, TAVR is nowadays also indicated in intermediate-risk patients as an alternative for surgical aortic valve replacement (SAVR). With the non-inferiority or even superiority of TAVR over SAVR in low-risk patients, the indications for TAVR are continuously expanding.1–3
One potential shortcoming of TAVR though is paravalvular regurgitation (PVR). The occurrence of PVR is common after TAVR, with incidences of mild PVR up to 40%, and incidences of greater than or equal to moderate PVR up to 10% in contemporary TAVR studies.4,5 In patients with greater than or equal to moderate PVR, mortality is three times higher compared to patients with none to trace PVR.6,7 Recently, also mild–moderate PVR was shown to be associated with an increased risk of mortality at 5 years after TAVR.8 Therefore, it is important to grade PVR during the procedure, both for prognostic and therapeutic purposes. Identification of relevant PVR during the TAVR procedure could guide additional interventions (e.g. post-dilation) to reduce PVR and improve patient outcome.
Adoption of the ‘minimalist TAVR’ approach has precluded the use of peri-procedural transoesophageal echocardiography (TEE) in procedural detection of PVR. Transthoracic echocardiography (TTE) has several limitations in the assessment of PVR, potentially underestimating the degree of PVR when compared with gold standard cardiac magnetic resonance (CMR).9,10 Angiographic grading is well feasible during the procedure but lacks diagnostic accuracy.11
Alternatively, haemodynamic indices to estimate PVR can be derived from transvalvular pressure tracings post-TAVR. Such transvalvular pressure tracings are used to assess remaining pressure gradients over the aortic valve prosthesis.12 Hence, these pressure tracings do not require additional instrumentation and do not involve additional contrast or radiation exposure. Commonly used haemodynamic indices to estimate PVR are diastolic delta (DD), heart rate-adjusted diastolic delta (HR-DD), aortic regurgitation index (ARI), and aortic regurgitation index ratio (ARI ratio). The latter three are in fact derivatives of DD, which is computed as the difference between end-diastolic blood pressure and left ventricular end-diastolic pressure (LVEDP). All four haemodynamic indices are correlated to mortality after TAVR.13–16 However, the predictive value of these indices for relevant PVR, in which gold standard CMR is used as the reference modality, has never been determined.
Therefore, the aim of the Assessment of Paravalvular Regurgitation After Transcatheter Aortic Valve Replacement by Hemodynamic Measurements and Cardiac Magnetic Resonance (APPOSE) trial was to assess the predictive value of peri-procedural haemodynamic indices for the occurrence of relevant PVR at 1 month follow-up as quantified by CMR.
Methods
Population and design
In this prospective single-centre study, we included consecutive patients who underwent a transfemoral or transaxillary TAVR for severe symptomatic AS at the Radboud University Medical Centre. In all patients, a self-expanding valve (Portico; Abbott Structural Heart, Minneapolis, MN, USA) was implanted, with valve sizes ranging between 23 and 29 mm. The main exclusion criteria were the presence of a pre-existing prosthetic cardiac device or valve, left ventricular ejection fraction (LVEF) <30%, and a serum creatinine >250 µmol/L or end-stage renal disease. The complete list of inclusion and exclusion criteria is provided in Supplementary data online, Table S1. The study protocol was approved by the local Medical Research Ethics Committee and by the institutional review board of the Radboud University Medical Centre. Written informed consent was obtained from all patients prior to inclusion. The APPOSE trial is registered in the ClinicalTrials.gov database (NCT04281771). This trial was funded by a research grant from Abbott. The sponsor of the trial had no role in the design and conduct of the trial; in the enrolment of participants; in the collection, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
TAVR procedure
All patients were discussed in a multi-disciplinary Heart Team and were deemed eligible for a TAVR procedure. TAVR was performed according to routine protocol. Procedures were performed in a hybrid catheterization laboratory with a standard operating team consisting of an interventional cardiologist, cardiothoracic surgeon, and anaesthesiologist. TAVR was performed either under general anaesthesia or under conscious sedation, which was left to the discretion of the operating team.
Haemodynamic measurements
We used 5F fluid-filled pigtail catheters (Impulse; Boston Scientific, Marlborough, MA, USA) to simultaneously measure continuous pressure in the aorta and the left ventricle. Heart rate was derived from continuous electrocardiographic monitoring. Haemodynamic data were displayed and stored in Mac-Lab (GE Healthcare, Chicago, IL, USA).
Haemodynamic measurements were performed at baseline (i.e. before pre-dilation), directly after implantation of the bioprosthetic valve, and (when applicable) after post-dilation. Data were captured and stored using Castor (Castor EDC, Amsterdam, the Netherlands) and exported for analysis to SPSS Statistics (version 25.0.0.1, IBM Corporation, Armonk, NY, USA). Haemodynamic data at baseline and after final TAVR result were used for the calculation of haemodynamic indices. For each haemodynamic index, the averaged pressures of at least three representative cardiac cycles during sinus rhythm (or paced rhythm) were used or at least five representative cycles during atrial fibrillation.
Haemodynamic indices were computed using the following calculations:
DD: end-diastolic blood pressure − LVEDP.
HR-DD: (DD/heart rate) * 80.
ARI: (DD/systolic blood pressure) * 100.
ARI ratio: ARI after implantation/ARI prior to implantation.
Echocardiographic assessment of PVR
TTE to determine the degree of PVR was performed 4–6 weeks after the TAVR procedure. Echocardiographic grading of PVR was based on an integrative multi-parametric approach that mainly included visual assessment of the number of PVR jets, jet width at the origin, and the circumferential extent of PVR. The degree of PVR was classified into none/trace, mild, moderate, or severe, according to Valve Academic Research Consortium-3 (VARC-3) criteria.17 Two researchers (M.R. and S.E.M.) independently assessed the echocardiographic degree of PVR. If consensus was not reached, a third researcher got involved (N.V.R.).
CMR measurements
All patients were scanned on the same day as the TTE assessment (4–6 weeks after TAVR) on a commercially available 1.5 T CMR scanner (Siemens Avanto; Siemens, Erlangen, Germany). Using a 2D phase-contrast velocity encoded spoiled gradient echo pulse sequence, the slice for the through-plane velocity quantification was placed perpendicular to the direction of the flow, just above the struts of the TAVR bioprosthesis. Flow acquisitions were performed during successive end-expiratory breath holds, using both a high velocity encoding (Venc) of at least 180 cm/s (or higher, depending on the velocity at which no aliasing was observed) and a low Venc of 75 cm/s. One cardiac cycle consisted of 25 phases. The high Venc was used for accurate assessment of the forward volume, the low Venc for determination of the regurgitant volume. CMR-derived regurgitant fraction (CMR-RF) was measured by dividing the regurgitant volume by the forward volume, multiplied by 100. The software package Medis Suite MR (Medis Medical Imaging, Leiden, the Netherlands) was used to analyse the CMR-RF.
Endpoints
The primary endpoint was the CMR-RF. Relevant PVR was defined as CMR-RF > 20%, whereas non-relevant PVR was defined as CMR-RF ≤ 20%.18 Secondary endpoints included procedural haemodynamic indices of PVR (DD, HR-DD, ARI, and ARI ratio) and short-term outcomes according to VARC-3 criteria.17
Statistical analysis
Haemodynamic indices with cut-offs previously associated with PVR were expected to be more prevalent in patients with greater than or equal to moderate PVR compared to patients with less than or equal to mild PVR. Based on previous literature, the prevalence of greater than or equal to moderate PVR was expected to be 30% in patients with levels of ARI above the median.19 Based on the information above, using χ2 test, a P value of 0.0125, and power of 80%, a total of 76 patients with analysable CMR scans and paired haemodynamic measurements were required. The sample size is comparable to previous studies in which CMR was compared with echocardiography in 71 patients20 and to aortic root angiography in 69 patients.11
Data are presented as mean ± standard deviation, median (interquartile range), or number (percentage), as appropriate. Baseline characteristics are displayed according to CMR-RF (CMR-RF > 20% vs. CMR-RF ≤ 20%). Categorical variables were compared using the χ2 test. Continuous variables were compared using Student’s t-test or Mann–Whitney U test, depending on normal distribution.
Correlation analysis was performed between the haemodynamic indices (DD, HR-DD, ARI, and ARI ratio) and CMR-RF, using Pearson’s correlation coefficient. Receiver operating characteristic (ROC) analyses were performed to assess the predictive value of the haemodynamic indices for CMR-RF > 20%. To allow ROC analysis, the continuous CMR-RF was dichotomized to CMR-RF > 20% and CMR-RF ≤ 20%.18 The area under the ROC curve (AUC) was used to quantify diagnostic performance of the haemodynamic indices, which were subsequently compared using DeLong’s test for correlated AUCs.21 Optimal cut-offs for all indices were defined as the cut-off value of the index at which the sensitivity (SE) and specificity (SP) curves intersect. When a higher SP was possible with the SE corresponding to the point of intersection, or vice versa, that cut-off value was chosen. Analyses were performed in SPSS Statistics (version 25.0.0.1, IBM Corporation, Armonk, NY, USA) and R (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Between November 2019 and October 2021, 103 consecutive patients who were accepted for TAVR provided written informed consent for the study. After exclusion of patients denoted as screen failure (n = 11) and patients without a complete CMR evaluation (n = 15), we examined a total of 77 patients (Figure 1). Baseline characteristics for patients with either CMR-RF > 20% or CMR-RF ≤ 20% are shown in Table 1. Mean age was 80.4 ± 5.1 years and 46.8% of patients were men. Median New York Heart Association (NYHA) function class was II, with 39.0% of patients being in NYHA class III or IV. Mean Society of Thoracic Surgeons (STS) and European System for Cardiac Operative Risk Evaluation (EuroSCORE) II were 2.38 ± 0.96 and 2.53 ± 1.63, respectively. Mean LVEF was 54.2 ± 8.4%, with a mean aortic valve area (AVA) and aortic valve mean gradient of 0.79 ± 0.18 cm2 and 45.2 ± 12.4 mmHg, respectively. No significant differences were found in baseline characteristics between patients with either CMR-RF > 20% or CMR-RF ≤ 20%.
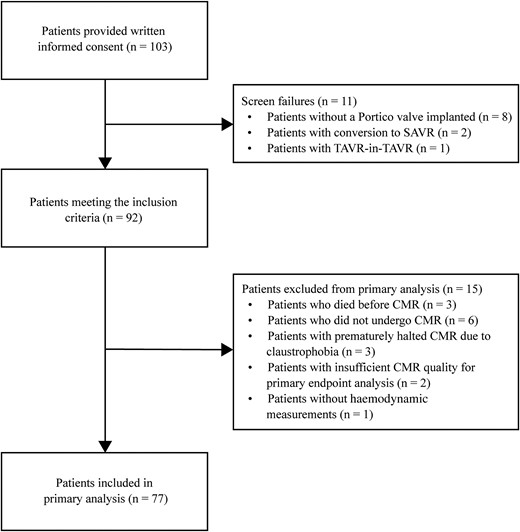
Study flowchart. CMR, cardiac magnetic resonance; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
| . | Study population (n = 77) . | CMR-RF ≤ 20% (n = 62) . | CMR-RF > 20% (n = 15) . | P value . |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 80.4 ± 5.1 | 80.5 ± 5.0 | 80.4 ± 5.9 | 0.972 |
| Male sex, n (%) | 36 (46.8) | 27 (43.5) | 9 (60) | 0.252 |
| Body mass index (BMI), kg/m2 | 27.3 ± 4.0 | 27.1 ± 4.0 | 28.1 ± 4.1 | 0.402 |
| Obesity, n (%) | 17 (22.1) | 12 (19.4) | 5 (33.3) | 0.300 |
| Smoker, n (%) | 5 (6.5) | 3 (4.8) | 2 (13.3) | 0.249 |
| Medical history | ||||
| STS score | 2.38 ± 0.96 | 2.38 ± 0.93 | 2.35 ± 1.11 | 0.893 |
| NYHA class | 2 [2–3] | 2 [2–3] | 2 [2–3] | 0.928 |
| NYHA class III/IV, n (%) | 30 (39.0) | 24 (38.8) | 6 (40.0) | 0.927 |
| Diabetes mellitus, n (%) | 20 (26.0) | 13 (21.0) | 7 (46.7) | 0.054 |
| Coronary artery disease, n (%) | 41 (53.2) | 33 (53.2) | 8 (53.3) | 0.994 |
| COPD, n (%) | 10 (13.0) | 8 (12.9) | 2 (13.3) | 1.000 |
| Atrial fibrillation, n (%) | 17 (22.1) | 14 (22.6) | 3 (20.0) | 1.000 |
| MDRD-GFR, mL/min | 64.8 ± 17.5 | 66.7 ± 17.0 | 57.0 ± 17.8 | 0.053 |
| Haemoglobin level, mmol/L | 7.9 ± 0.9 | 8.0 ± 0.9 | 7.6 ± 1.0 | 0.122 |
| Pre-procedural echocardiographic parameters | ||||
| Aortic valve area, cm2 | 0.79 ± 0.18 | 0.78 ± 0.19 | 0.80 ± 0.11 | 0.794 |
| Aortic valve mean gradient, mmHg | 45.2 ± 12.4 | 44.9 ± 11.6 | 46.5 ± 15.7 | 0.642 |
| Aortic valve maximum velocity, m/s | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.3 ± 0.6 | 0.583 |
| Left ventricular ejection fraction, % | 54.2 ± 8.4 | 54.8 ± 8.3 | 51.7 ± 8.4 | 0.203 |
| Moderate or severe aortic regurgitation, n (%) | 7 (9.1) | 6 (9.7) | 1 (6.7) | 1.000 |
| . | Study population (n = 77) . | CMR-RF ≤ 20% (n = 62) . | CMR-RF > 20% (n = 15) . | P value . |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 80.4 ± 5.1 | 80.5 ± 5.0 | 80.4 ± 5.9 | 0.972 |
| Male sex, n (%) | 36 (46.8) | 27 (43.5) | 9 (60) | 0.252 |
| Body mass index (BMI), kg/m2 | 27.3 ± 4.0 | 27.1 ± 4.0 | 28.1 ± 4.1 | 0.402 |
| Obesity, n (%) | 17 (22.1) | 12 (19.4) | 5 (33.3) | 0.300 |
| Smoker, n (%) | 5 (6.5) | 3 (4.8) | 2 (13.3) | 0.249 |
| Medical history | ||||
| STS score | 2.38 ± 0.96 | 2.38 ± 0.93 | 2.35 ± 1.11 | 0.893 |
| NYHA class | 2 [2–3] | 2 [2–3] | 2 [2–3] | 0.928 |
| NYHA class III/IV, n (%) | 30 (39.0) | 24 (38.8) | 6 (40.0) | 0.927 |
| Diabetes mellitus, n (%) | 20 (26.0) | 13 (21.0) | 7 (46.7) | 0.054 |
| Coronary artery disease, n (%) | 41 (53.2) | 33 (53.2) | 8 (53.3) | 0.994 |
| COPD, n (%) | 10 (13.0) | 8 (12.9) | 2 (13.3) | 1.000 |
| Atrial fibrillation, n (%) | 17 (22.1) | 14 (22.6) | 3 (20.0) | 1.000 |
| MDRD-GFR, mL/min | 64.8 ± 17.5 | 66.7 ± 17.0 | 57.0 ± 17.8 | 0.053 |
| Haemoglobin level, mmol/L | 7.9 ± 0.9 | 8.0 ± 0.9 | 7.6 ± 1.0 | 0.122 |
| Pre-procedural echocardiographic parameters | ||||
| Aortic valve area, cm2 | 0.79 ± 0.18 | 0.78 ± 0.19 | 0.80 ± 0.11 | 0.794 |
| Aortic valve mean gradient, mmHg | 45.2 ± 12.4 | 44.9 ± 11.6 | 46.5 ± 15.7 | 0.642 |
| Aortic valve maximum velocity, m/s | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.3 ± 0.6 | 0.583 |
| Left ventricular ejection fraction, % | 54.2 ± 8.4 | 54.8 ± 8.3 | 51.7 ± 8.4 | 0.203 |
| Moderate or severe aortic regurgitation, n (%) | 7 (9.1) | 6 (9.7) | 1 (6.7) | 1.000 |
Data are presented as mean ± standard deviation, median [interquartile range], or as number (%).
CMR-RF, cardiac magnetic resonance-regurgitant fraction; COPD, chronic obstructive pulmonary disease; MDRD-GFR, Modification of Diet in Renal Disease—glomerular filtration rate; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
| . | Study population (n = 77) . | CMR-RF ≤ 20% (n = 62) . | CMR-RF > 20% (n = 15) . | P value . |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 80.4 ± 5.1 | 80.5 ± 5.0 | 80.4 ± 5.9 | 0.972 |
| Male sex, n (%) | 36 (46.8) | 27 (43.5) | 9 (60) | 0.252 |
| Body mass index (BMI), kg/m2 | 27.3 ± 4.0 | 27.1 ± 4.0 | 28.1 ± 4.1 | 0.402 |
| Obesity, n (%) | 17 (22.1) | 12 (19.4) | 5 (33.3) | 0.300 |
| Smoker, n (%) | 5 (6.5) | 3 (4.8) | 2 (13.3) | 0.249 |
| Medical history | ||||
| STS score | 2.38 ± 0.96 | 2.38 ± 0.93 | 2.35 ± 1.11 | 0.893 |
| NYHA class | 2 [2–3] | 2 [2–3] | 2 [2–3] | 0.928 |
| NYHA class III/IV, n (%) | 30 (39.0) | 24 (38.8) | 6 (40.0) | 0.927 |
| Diabetes mellitus, n (%) | 20 (26.0) | 13 (21.0) | 7 (46.7) | 0.054 |
| Coronary artery disease, n (%) | 41 (53.2) | 33 (53.2) | 8 (53.3) | 0.994 |
| COPD, n (%) | 10 (13.0) | 8 (12.9) | 2 (13.3) | 1.000 |
| Atrial fibrillation, n (%) | 17 (22.1) | 14 (22.6) | 3 (20.0) | 1.000 |
| MDRD-GFR, mL/min | 64.8 ± 17.5 | 66.7 ± 17.0 | 57.0 ± 17.8 | 0.053 |
| Haemoglobin level, mmol/L | 7.9 ± 0.9 | 8.0 ± 0.9 | 7.6 ± 1.0 | 0.122 |
| Pre-procedural echocardiographic parameters | ||||
| Aortic valve area, cm2 | 0.79 ± 0.18 | 0.78 ± 0.19 | 0.80 ± 0.11 | 0.794 |
| Aortic valve mean gradient, mmHg | 45.2 ± 12.4 | 44.9 ± 11.6 | 46.5 ± 15.7 | 0.642 |
| Aortic valve maximum velocity, m/s | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.3 ± 0.6 | 0.583 |
| Left ventricular ejection fraction, % | 54.2 ± 8.4 | 54.8 ± 8.3 | 51.7 ± 8.4 | 0.203 |
| Moderate or severe aortic regurgitation, n (%) | 7 (9.1) | 6 (9.7) | 1 (6.7) | 1.000 |
| . | Study population (n = 77) . | CMR-RF ≤ 20% (n = 62) . | CMR-RF > 20% (n = 15) . | P value . |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 80.4 ± 5.1 | 80.5 ± 5.0 | 80.4 ± 5.9 | 0.972 |
| Male sex, n (%) | 36 (46.8) | 27 (43.5) | 9 (60) | 0.252 |
| Body mass index (BMI), kg/m2 | 27.3 ± 4.0 | 27.1 ± 4.0 | 28.1 ± 4.1 | 0.402 |
| Obesity, n (%) | 17 (22.1) | 12 (19.4) | 5 (33.3) | 0.300 |
| Smoker, n (%) | 5 (6.5) | 3 (4.8) | 2 (13.3) | 0.249 |
| Medical history | ||||
| STS score | 2.38 ± 0.96 | 2.38 ± 0.93 | 2.35 ± 1.11 | 0.893 |
| NYHA class | 2 [2–3] | 2 [2–3] | 2 [2–3] | 0.928 |
| NYHA class III/IV, n (%) | 30 (39.0) | 24 (38.8) | 6 (40.0) | 0.927 |
| Diabetes mellitus, n (%) | 20 (26.0) | 13 (21.0) | 7 (46.7) | 0.054 |
| Coronary artery disease, n (%) | 41 (53.2) | 33 (53.2) | 8 (53.3) | 0.994 |
| COPD, n (%) | 10 (13.0) | 8 (12.9) | 2 (13.3) | 1.000 |
| Atrial fibrillation, n (%) | 17 (22.1) | 14 (22.6) | 3 (20.0) | 1.000 |
| MDRD-GFR, mL/min | 64.8 ± 17.5 | 66.7 ± 17.0 | 57.0 ± 17.8 | 0.053 |
| Haemoglobin level, mmol/L | 7.9 ± 0.9 | 8.0 ± 0.9 | 7.6 ± 1.0 | 0.122 |
| Pre-procedural echocardiographic parameters | ||||
| Aortic valve area, cm2 | 0.79 ± 0.18 | 0.78 ± 0.19 | 0.80 ± 0.11 | 0.794 |
| Aortic valve mean gradient, mmHg | 45.2 ± 12.4 | 44.9 ± 11.6 | 46.5 ± 15.7 | 0.642 |
| Aortic valve maximum velocity, m/s | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.3 ± 0.6 | 0.583 |
| Left ventricular ejection fraction, % | 54.2 ± 8.4 | 54.8 ± 8.3 | 51.7 ± 8.4 | 0.203 |
| Moderate or severe aortic regurgitation, n (%) | 7 (9.1) | 6 (9.7) | 1 (6.7) | 1.000 |
Data are presented as mean ± standard deviation, median [interquartile range], or as number (%).
CMR-RF, cardiac magnetic resonance-regurgitant fraction; COPD, chronic obstructive pulmonary disease; MDRD-GFR, Modification of Diet in Renal Disease—glomerular filtration rate; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Procedural characteristics and clinical outcomes
Details of the procedural characteristics and clinical outcomes are provided in Table 2. A total of 17 (22.1%) TAVR procedures were performed under general anaesthesia, and 60 (77.9%) patients underwent TAVR under conscious sedation. In 70 (90.9%) patients, the transfemoral approach was used, and the transaxillary approach was used in the remaining seven (9.1%) patients. Pre-dilation was performed in 97.4% of all TAVR procedures, and post-dilation was performed in 19.5% of patients. A second valve was implanted in two (2.6%) patients due to migration of the first TAVR bioprosthesis. Median implantation depth below aortic annulus was 4 mm (IQR 4–6).
| . | Study population (n = 77) . |
|---|---|
| Procedural characteristics | |
| Anaesthesia | |
| General anaesthesia, n (%) | 17 (22.1) |
| Conscious sedation, n (%) | 60 (77.9) |
| Approach | |
| Transfemoral, n (%) | 70 (90.9) |
| Transaxillary, n (%) | 7 (9.1) |
| Pre-dilation, n (%) | 75 (97.4) |
| Post-dilation, n (%) | 15 (19.5) |
| Implantation depth, millimetre below aortic annulus | 4 [4–6] |
| Second valve implanted, n (%) | 2 (2.6) |
| Clinical outcomes | |
| All stroke at 30 days, n (%) | 3 (3.9) |
| Stroke, n (%) | 2 (2.6) |
| TIA, n (%) | 1 (1.3) |
| Acute kidney injury, n (%) | 1 (1.3) |
| Permanent pacemaker implantation < 30 days, n (%) | 8 (10.3) |
| Vascular complications < 30 days, n (%) | 13 (16.9) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 11 (14.3) |
| Bleeding complications < 30 days, n (%) | 11 (14.3) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 9 (11.7) |
| Technical success (at exit from procedure room), n (%) | 74 (96.1) |
| Device success (at 30 days), n (%) | 73 (94.8) |
| Early safety (at 30 days), n (%) | 66 (85.7) |
| . | Study population (n = 77) . |
|---|---|
| Procedural characteristics | |
| Anaesthesia | |
| General anaesthesia, n (%) | 17 (22.1) |
| Conscious sedation, n (%) | 60 (77.9) |
| Approach | |
| Transfemoral, n (%) | 70 (90.9) |
| Transaxillary, n (%) | 7 (9.1) |
| Pre-dilation, n (%) | 75 (97.4) |
| Post-dilation, n (%) | 15 (19.5) |
| Implantation depth, millimetre below aortic annulus | 4 [4–6] |
| Second valve implanted, n (%) | 2 (2.6) |
| Clinical outcomes | |
| All stroke at 30 days, n (%) | 3 (3.9) |
| Stroke, n (%) | 2 (2.6) |
| TIA, n (%) | 1 (1.3) |
| Acute kidney injury, n (%) | 1 (1.3) |
| Permanent pacemaker implantation < 30 days, n (%) | 8 (10.3) |
| Vascular complications < 30 days, n (%) | 13 (16.9) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 11 (14.3) |
| Bleeding complications < 30 days, n (%) | 11 (14.3) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 9 (11.7) |
| Technical success (at exit from procedure room), n (%) | 74 (96.1) |
| Device success (at 30 days), n (%) | 73 (94.8) |
| Early safety (at 30 days), n (%) | 66 (85.7) |
Data are presented as mean ± standard deviation, median [interquartile range], or as number (%).
TIA, transient ischemic attack.
| . | Study population (n = 77) . |
|---|---|
| Procedural characteristics | |
| Anaesthesia | |
| General anaesthesia, n (%) | 17 (22.1) |
| Conscious sedation, n (%) | 60 (77.9) |
| Approach | |
| Transfemoral, n (%) | 70 (90.9) |
| Transaxillary, n (%) | 7 (9.1) |
| Pre-dilation, n (%) | 75 (97.4) |
| Post-dilation, n (%) | 15 (19.5) |
| Implantation depth, millimetre below aortic annulus | 4 [4–6] |
| Second valve implanted, n (%) | 2 (2.6) |
| Clinical outcomes | |
| All stroke at 30 days, n (%) | 3 (3.9) |
| Stroke, n (%) | 2 (2.6) |
| TIA, n (%) | 1 (1.3) |
| Acute kidney injury, n (%) | 1 (1.3) |
| Permanent pacemaker implantation < 30 days, n (%) | 8 (10.3) |
| Vascular complications < 30 days, n (%) | 13 (16.9) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 11 (14.3) |
| Bleeding complications < 30 days, n (%) | 11 (14.3) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 9 (11.7) |
| Technical success (at exit from procedure room), n (%) | 74 (96.1) |
| Device success (at 30 days), n (%) | 73 (94.8) |
| Early safety (at 30 days), n (%) | 66 (85.7) |
| . | Study population (n = 77) . |
|---|---|
| Procedural characteristics | |
| Anaesthesia | |
| General anaesthesia, n (%) | 17 (22.1) |
| Conscious sedation, n (%) | 60 (77.9) |
| Approach | |
| Transfemoral, n (%) | 70 (90.9) |
| Transaxillary, n (%) | 7 (9.1) |
| Pre-dilation, n (%) | 75 (97.4) |
| Post-dilation, n (%) | 15 (19.5) |
| Implantation depth, millimetre below aortic annulus | 4 [4–6] |
| Second valve implanted, n (%) | 2 (2.6) |
| Clinical outcomes | |
| All stroke at 30 days, n (%) | 3 (3.9) |
| Stroke, n (%) | 2 (2.6) |
| TIA, n (%) | 1 (1.3) |
| Acute kidney injury, n (%) | 1 (1.3) |
| Permanent pacemaker implantation < 30 days, n (%) | 8 (10.3) |
| Vascular complications < 30 days, n (%) | 13 (16.9) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 11 (14.3) |
| Bleeding complications < 30 days, n (%) | 11 (14.3) |
| Major, n (%) | 2 (2.6) |
| Minor, n (%) | 9 (11.7) |
| Technical success (at exit from procedure room), n (%) | 74 (96.1) |
| Device success (at 30 days), n (%) | 73 (94.8) |
| Early safety (at 30 days), n (%) | 66 (85.7) |
Data are presented as mean ± standard deviation, median [interquartile range], or as number (%).
TIA, transient ischemic attack.
Within 30 days of the procedure, stroke/TIA occurred in three (3.9%) patients, permanent pacemaker implantation in eight (10.3%) patients, major vascular complications in two (2.6%) patients, and major bleeding complications in two (2.6%) patients. Acute kidney injury requiring temporary dialysis occurred in one (1.3%) patient. Technical success was 96.1%, and device success was 94.8%.
Haemodynamic measurements
Procedural haemodynamic measurements are presented in Table 3. Pre-implantation, mean heart rate was 67 ± 15 BPM, LVEDP was 16 ± 6 mmHg, and peak-to-peak gradient was 53 ± 17 mmHg. Mean DD was 38 ± 11 mmHg, HR-DD was 47 ± 14 mmHg/BPM, and ARI was 33 ± 9. Except for DD, all parameters changed significantly after implantation. Mean heart rate increased to 78 ± 18 BPM (P < 0.001), LVEDP increased to 19 ± 6 mmHg (P = 0.010), and peak-to-peak gradient decreased to 4 ± 3 mmHg (P < 0.001). HR-DD decreased to 42 ± 13 mmHg/BPM (P = 0.001), and ARI decreased to 29 ± 9 (P = 0.003). Mean ARI ratio was 0.95 ± 0.45.
| . | Pre-implantation . | Post-implantation . | P value . |
|---|---|---|---|
| Heart rate, beats per minute (BPM) | 67 ± 15 | 78 ± 18 | <0.001 |
| Systolic blood pressure (SBP), mmHg | 118 ± 22 | 140 ± 28 | <0.001 |
| Diastolic blood pressure (DBP), mmHg | 54 ± 10 | 58 ± 9 | 0.006 |
| Left ventricular systolic pressure (LVSP), mmHg | 171 ± 27 | 144 ± 27 | <0.001 |
| Left ventricular end-diastolic pressure (LVEDP), mmHg | 16 ± 6 | 19 ± 6 | 0.010 |
| Peak–peak gradient, mmHg | 53 ± 17 | 4 ± 3 | <0.001 |
| Mean gradient, mmHg | 47 ± 12 | 9 ± 4 | <0.001 |
| Haemodynamic indices | |||
| Diastolic delta (DD) | 38 ± 11 | 39 ± 11 | 0.385 |
| Heart rate-adjusted diastolic delta (HR-DD) | 47 ± 14 | 42 ± 13 | 0.001 |
| Aortic regurgitation index (ARI) | 33 ± 9 | 29 ± 9 | 0.003 |
| Aortic regurgitation index ratio (ARI ratio) | N/A | 0.95 ± 0.45 | N/A |
| . | Pre-implantation . | Post-implantation . | P value . |
|---|---|---|---|
| Heart rate, beats per minute (BPM) | 67 ± 15 | 78 ± 18 | <0.001 |
| Systolic blood pressure (SBP), mmHg | 118 ± 22 | 140 ± 28 | <0.001 |
| Diastolic blood pressure (DBP), mmHg | 54 ± 10 | 58 ± 9 | 0.006 |
| Left ventricular systolic pressure (LVSP), mmHg | 171 ± 27 | 144 ± 27 | <0.001 |
| Left ventricular end-diastolic pressure (LVEDP), mmHg | 16 ± 6 | 19 ± 6 | 0.010 |
| Peak–peak gradient, mmHg | 53 ± 17 | 4 ± 3 | <0.001 |
| Mean gradient, mmHg | 47 ± 12 | 9 ± 4 | <0.001 |
| Haemodynamic indices | |||
| Diastolic delta (DD) | 38 ± 11 | 39 ± 11 | 0.385 |
| Heart rate-adjusted diastolic delta (HR-DD) | 47 ± 14 | 42 ± 13 | 0.001 |
| Aortic regurgitation index (ARI) | 33 ± 9 | 29 ± 9 | 0.003 |
| Aortic regurgitation index ratio (ARI ratio) | N/A | 0.95 ± 0.45 | N/A |
Data are presented as mean ± standard deviation.
N/A, not applicable.
| . | Pre-implantation . | Post-implantation . | P value . |
|---|---|---|---|
| Heart rate, beats per minute (BPM) | 67 ± 15 | 78 ± 18 | <0.001 |
| Systolic blood pressure (SBP), mmHg | 118 ± 22 | 140 ± 28 | <0.001 |
| Diastolic blood pressure (DBP), mmHg | 54 ± 10 | 58 ± 9 | 0.006 |
| Left ventricular systolic pressure (LVSP), mmHg | 171 ± 27 | 144 ± 27 | <0.001 |
| Left ventricular end-diastolic pressure (LVEDP), mmHg | 16 ± 6 | 19 ± 6 | 0.010 |
| Peak–peak gradient, mmHg | 53 ± 17 | 4 ± 3 | <0.001 |
| Mean gradient, mmHg | 47 ± 12 | 9 ± 4 | <0.001 |
| Haemodynamic indices | |||
| Diastolic delta (DD) | 38 ± 11 | 39 ± 11 | 0.385 |
| Heart rate-adjusted diastolic delta (HR-DD) | 47 ± 14 | 42 ± 13 | 0.001 |
| Aortic regurgitation index (ARI) | 33 ± 9 | 29 ± 9 | 0.003 |
| Aortic regurgitation index ratio (ARI ratio) | N/A | 0.95 ± 0.45 | N/A |
| . | Pre-implantation . | Post-implantation . | P value . |
|---|---|---|---|
| Heart rate, beats per minute (BPM) | 67 ± 15 | 78 ± 18 | <0.001 |
| Systolic blood pressure (SBP), mmHg | 118 ± 22 | 140 ± 28 | <0.001 |
| Diastolic blood pressure (DBP), mmHg | 54 ± 10 | 58 ± 9 | 0.006 |
| Left ventricular systolic pressure (LVSP), mmHg | 171 ± 27 | 144 ± 27 | <0.001 |
| Left ventricular end-diastolic pressure (LVEDP), mmHg | 16 ± 6 | 19 ± 6 | 0.010 |
| Peak–peak gradient, mmHg | 53 ± 17 | 4 ± 3 | <0.001 |
| Mean gradient, mmHg | 47 ± 12 | 9 ± 4 | <0.001 |
| Haemodynamic indices | |||
| Diastolic delta (DD) | 38 ± 11 | 39 ± 11 | 0.385 |
| Heart rate-adjusted diastolic delta (HR-DD) | 47 ± 14 | 42 ± 13 | 0.001 |
| Aortic regurgitation index (ARI) | 33 ± 9 | 29 ± 9 | 0.003 |
| Aortic regurgitation index ratio (ARI ratio) | N/A | 0.95 ± 0.45 | N/A |
Data are presented as mean ± standard deviation.
N/A, not applicable.
Echocardiographic assessment of PVR
Mean duration between TAVR and TTE was 41 ± 14 days. TTE assessment showed none/trace PVR in 41 (53.2%) patients, mild PVR in 34 (44.2%) patients, moderate PVR in two (2.6%) patients, and no patients with severe PVR.
CMR quantification of regurgitant fraction (CMR-RF)
Mean forward volume measured with a high (≥180 cm/s) velocity ending (Venc) was 77.8 ± 19.0 mL. Mean regurgitant volume measured with a low Venc (75 cm/s) was 10.1 ± 8.5 mL, resulting in a mean regurgitant fraction of 12.4 ± 9.3%. Fifteen (19.5%) patients had CMR-RF > 20% (Table 4).
Cardiac magnetic resonance (CMR) and echocardiographic assessment of paravalvular regurgitation (PVR)
| . | Study population (n = 77) . |
|---|---|
| Days after TAVR | 41 ± 14 |
| Flow measurements | |
| Forward volume, mL | 77.8 ± 19.0 |
| Regurgitant volume, mL | 10.1 ± 8.5 |
| Regurgitant fraction, % | 12.4 ± 9.3 |
| CMR-RF classification | |
| CMR-RF > 20%, n (%) | 15 (19.5) |
| CMR-RF ≤ 20%, n (%) | 62 (80.5) |
| TTE classification of PVRa | |
| None/trace, n (%) | 41 (53.2) |
| Mild, n (%) | 34 (44.2) |
| Moderate, n (%) | 2 (2.6) |
| Severe, n (%) | 0 |
| . | Study population (n = 77) . |
|---|---|
| Days after TAVR | 41 ± 14 |
| Flow measurements | |
| Forward volume, mL | 77.8 ± 19.0 |
| Regurgitant volume, mL | 10.1 ± 8.5 |
| Regurgitant fraction, % | 12.4 ± 9.3 |
| CMR-RF classification | |
| CMR-RF > 20%, n (%) | 15 (19.5) |
| CMR-RF ≤ 20%, n (%) | 62 (80.5) |
| TTE classification of PVRa | |
| None/trace, n (%) | 41 (53.2) |
| Mild, n (%) | 34 (44.2) |
| Moderate, n (%) | 2 (2.6) |
| Severe, n (%) | 0 |
Data are presented as mean ± standard deviation or as number (%).
CMR-RF, cardiac magnetic resonance-regurgitant fraction; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiography.
TTE was performed on the same day as CMR.
Cardiac magnetic resonance (CMR) and echocardiographic assessment of paravalvular regurgitation (PVR)
| . | Study population (n = 77) . |
|---|---|
| Days after TAVR | 41 ± 14 |
| Flow measurements | |
| Forward volume, mL | 77.8 ± 19.0 |
| Regurgitant volume, mL | 10.1 ± 8.5 |
| Regurgitant fraction, % | 12.4 ± 9.3 |
| CMR-RF classification | |
| CMR-RF > 20%, n (%) | 15 (19.5) |
| CMR-RF ≤ 20%, n (%) | 62 (80.5) |
| TTE classification of PVRa | |
| None/trace, n (%) | 41 (53.2) |
| Mild, n (%) | 34 (44.2) |
| Moderate, n (%) | 2 (2.6) |
| Severe, n (%) | 0 |
| . | Study population (n = 77) . |
|---|---|
| Days after TAVR | 41 ± 14 |
| Flow measurements | |
| Forward volume, mL | 77.8 ± 19.0 |
| Regurgitant volume, mL | 10.1 ± 8.5 |
| Regurgitant fraction, % | 12.4 ± 9.3 |
| CMR-RF classification | |
| CMR-RF > 20%, n (%) | 15 (19.5) |
| CMR-RF ≤ 20%, n (%) | 62 (80.5) |
| TTE classification of PVRa | |
| None/trace, n (%) | 41 (53.2) |
| Mild, n (%) | 34 (44.2) |
| Moderate, n (%) | 2 (2.6) |
| Severe, n (%) | 0 |
Data are presented as mean ± standard deviation or as number (%).
CMR-RF, cardiac magnetic resonance-regurgitant fraction; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiography.
TTE was performed on the same day as CMR.
Relation between haemodynamic indices and CMR-RF
Correlation analysis showed that DD had the best (inverse) correlation with CMR-RF (correlation coefficient: −0.352, P = 0.002), followed by HR-DD (correlation coefficient: −0.344, P = 0.002), ARI (correlation coefficient: −0.251 P = 0.027), and ARI ratio (correlation coefficient: −0.145, P = 0.209). DD also had the highest AUC (0.82; 95% CI, 0.72–0.92), followed by HR-DD (AUC 0.78; 95% CI, 0.67–0.89), ARI (AUC 0.78; 95% CI, 0.66–0.89), and ARI ratio (AUC 0.65; 95% CI, 0.49–0.81) (DD vs. ARI ratio, P = 0.01, other comparisons between AUCs non-significant) (Figure 2). Optimization of the cut-offs resulted in the following values: 32 mmHg for DD (SE: 69%, SP: 77%), 37 mmHg/BPM for HR-DD (SE: 69%, SP: 67%), 25 for ARI (SE: 69%, SP: 69%), and 0.85 for ARI ratio (SE: 62%, SP: 58%). The previously proposed cut-off of ≤18 mmHg for DD had a SE of 0% and SP of 98% for the detection of CMR-RF > 20%. The SE and SP curves for DD to detect CMR-RF > 20% are shown in Figure 3.
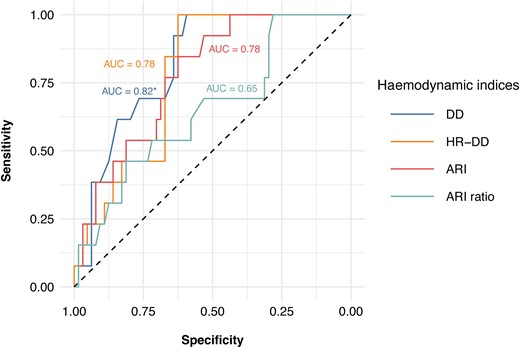
ROC analyses showing correlation between haemodynamic indices and relevant PVR expressed as CMR-RF > 20%. ARI, aortic regurgitation index; ARI ratio, aortic regurgitation index ratio; AUC, area under the curve; CMR-RF, cardiac magnetic resonance-regurgitant fraction; DD, diastolic delta; HR-DD, heart rate-adjusted diastolic delta; PVR, paravalvular regurgitation; Venc, velocity encoding. Asterisk (*) indicates statistically significant difference between AUCs of DD and ARI ratio (P = 0.01).
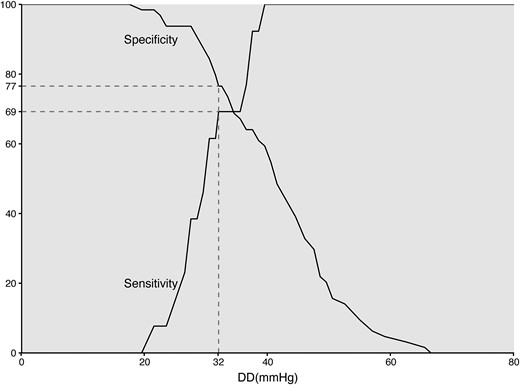
Sensitivity and specificity curves of DD to detect relevant PVR expressed as CMR-RF > 20%. CMR-RF, cardiac magnetic resonance-regurgitant fraction; DD, diastolic delta; PVR, paravalvular regurgitation.
Discussion
In this study, we assessed four haemodynamic indices for their predictive value for relevant PVR based on gold standard CMR. This imaging modality served as the reference standard, in order to identify the most accurate haemodynamic index to grade PVR.
The key findings can be summarized as follows. First, in this prospective non-selective cohort of consecutive TAVR patients, we found a mean CMR-RF of 12%, with 19.5% of patients showing a CMR-RF > 20%. Second, the haemodynamic index DD had the best correlation with CMR-RF and the highest predictive value for CMR-RF > 20%, followed by HR-DD, ARI, and ARI ratio. Third, a cut-off value of 32 mmHg for DD provides a SE of 69% and a SP of 77% for the presence of CMR-RF > 20% at 1-month follow-up.
The mean CMR-RF of 12% in our study is comparable to the CMR-RF of other TAVR devices.9,11 Echocardiographic assessment of PVR at 1-month follow-up also demonstrated that the majority of patients in our study had none/trace PVR, with only two patients with moderate PVR, and no cases of severe PVR. These numbers are in line with previous studies reporting on the echocardiographic performance of the Abbott Portico valve, as well as other TAVR platforms.22,23
A variety of CMR classifications have been proposed in literature, using different cut-offs to grade PVR. In a study performed by Ribeiro et al.,24 it was shown that a CMR-RF ≥ 30% was associated with an increased risk of mortality and the composite of mortality and rehospitalization for heart failure. In the 2022 EACVI and ESC position paper on multi-modality imaging assessment of native valvular regurgitation, the CMR cut-offs for mild, moderate, and severe aortic regurgitation are <30%, 30–49%, and ≥50%, respectively.25 Using this CMR grading scheme in our cohort would categorize 74 (96.1%) patients into less than or equal to mild PVR, three (3.9%) patients into moderate PVR, and zero patients into severe PVR. This closely resembles the degree of PVR as assessed with TTE (see Supplementary data online, Table S2). However, others have shown negative impact on clinical outcome at lower cut-offs.18 Thus, for the present study, we adopted the more conservative CMR-RF cut-off value of 20%, aiming to diminish the risk of missing patients with potentially clinically relevant PVR. Another argument to use a more conservative cut-off in the present setting is the fact that a very high degree of PVR is generally already observed by angiography, thereby reducing the additional value of haemodynamic indices.
Although the incidences of mild and greater than or equal to moderate PVR as assessed by echocardiography have decreased with newer-generation TAVR devices and advancing techniques, risk of PVR is still considered a major shortcoming of TAVR. Moderate or severe PVR strongly and negatively affects clinical outcome, as illustrated by a three-fold increase in all-cause and cardiovascular mortality after TAVR compared with patients with none/trace PVR.7,26,27 Recently, also mild–moderate PVR has been associated with an increased risk of mortality at 5 years after TAVR, implicating that also with this degree of PVR, additional interventions should be considered to reduce PVR.8
Moreover, we recently identified PVR as risk factor for recurrence of gastrointestinal bleeding after TAVR in patients with Heyde syndrome, further stressing the need for reduction of PVR.28 Thus, assessment of PVR, including the use of the correct parameters and/or adoption of sensitive cut-offs, is of utmost importance in determining prognosis. Also, it could guide additional therapies aiming to reduce PVR and improve clinical outcome after TAVR.
Treatment options for PVR include balloon post-dilation (BPD), transcatheter valve-in-valve (ViV), and percutaneous closure by a vascular plug. BPD is usually considered first since this is easily performed during TAVR procedure and particularly effective in case of inadequate apposition or relative under sizing of the valve. Although BPD is an effective strategy in reducing PVR, the incidence of stroke after BPD is significantly higher,29 and BPD can cause aortic rupture. Therefore, unnecessary BPD should be avoided. Transcatheter placement of a second valve (ViV) can adequately reduce significant PVR in case of malposition of the prosthesis (either too high or too low compared with the aortic annulus).30 Also, transcatheter closure of a paravalvular leak is feasible, although its use is still limited.31,32
Echocardiography also allows assessment of PVR, especially TEE. However, with the advent of the ‘minimalist TAVR’ approach, a commonly used term for performing TAVR under local anaesthesia with minimal sedation and promoting early discharge, the use of peri-procedural TEE as imaging modality is more or less precluded. TTE is less accurate in the assessment of PVR compared with TEE.33,34 Various patient factors such as airway disease and habitus often limit acoustic windows for TTE. Regurgitant jets are often multiple and eccentric, making traditional echocardiographic regurgitation assessment techniques invalid. As a result, TTE potentially underestimates the degree of PVR when compared with gold standard CMR.9,10,18
Angiographic assessment using the visual Sellers’ method also has several limitations, including subjectivity and lack of accuracy in quantifying PVR.34,35 Moreover, aortic root angiography correlates only moderately to CMR in the classification of PVR.11 This highlights the limitations of conventional modalities and the need for objective methodologies to aid in PVR assessment.
Transvalvular haemodynamic indices are cheap and readily available, can be performed at multiple instances during the procedure, and have very low inter- and intra-observer variability. Therefore, several studies have assessed the prognostic value of different peri-procedural haemodynamic indices. In these studies, it was shown that DD ≤ 18 mmHg,13 HR-DD < 25 mmHg/BPM,14 ARI < 25,15 and ARI ratio < 0.616 are all independently associated with (1-year) mortality after TAVR. Previously, we showed that ARI ratio < 0.6 is the strongest haemodynamic predictor of 1-year mortality after TAVR.36 In the present study, interestingly ARI ratio was least predictive for the occurrence of CMR-RF > 20%. Indeed, for clinical outcome not only the post-TAVR occurrence of PVR is important but also the pre-TAVR haemodynamic circumstances like diastolic dysfunction and pre-existing aortic regurgitation.37 Therefore, ARI ratio and DD provide different and supplemental information for guidance of therapeutic strategy and prognosis.
We found that, regarding DD, the best cut-off to predict CMR-RF > 20% was 32 mmHg. This implies that in patients with a DD ≤ 32 mmHg, it could be considered to perform additional post-dilation, especially when conventional imaging modalities like aortic root angiography or echocardiography point in the same direction. Naturally, this should be left to the treating physician, also considering the risks of additional post-dilation. Future studies need to validate such an approach.
HR-DD, ARI, and ARI ratio had lower AUCs than DD, with a significantly lower AUC for ARI ratio than for DD. The aforementioned parameters are derivatives of DD, necessitating an additional calculation compared with the DD. In other words, our findings suggest that the most simple and most readily available haemodynamic index yields the highest accuracy to predict CMR-RF > 20%.
Besides the four haemodynamic indices assessed in this study, additional haemodynamic parameters for assessment of PVR have been addressed in literature. Integration of the systolic and diastolic time components during measurement of the ARI has resulted in the time-integrated aortic regurgitation index (TIARI), which shows a higher AUC compared with the ARI,38 and can be useful to guide BPD after valve deployment.39 Dividing the area between aortic and left ventricular pressure-time curves by the duration of diastole generates the diastolic pressure-time index (DPTI). When DPTI is divided by the systolic blood pressure and multiplied by 100, the DPTI adjusted is obtained, which could be considered to differentiate between relevant and non-relevant PVR after TAVR.40 However, since both the TIARI and DPTI are time-integrated measures that are not readily available during TAVR procedure, we did not integrate these in the present study.
Future perspectives
Future studies need to be conducted to prospectively assess the newly proposed cut-offs for the haemodynamic parameters described in our study. The DD in particular can be used to aid peri-procedural decision-making. Furthermore, videodensitometry has emerged as a complementary modality for grading PVR. This technique shows a high correlation with CMR-RF and can be used both offline and online.41,42
Limitations
This trial has some limitations. First, the use of CMR might have led to selection bias whereby patients with an overall impaired health status could tend to decline participation in this study. Second, assessment of the accuracy of haemodynamic indices to predict a CMR-RF ≥ 30% was prohibited due to the limited number of patients in this group. Third, this study is solely performed with the self-expanding Abbott Portico bioprosthesis; therefore, the results are not directly applicable to other types of TAVR devices.
Conclusion
In conclusion, we have demonstrated that the diastolic delta has the highest predictive value for the occurrence of relevant PVR (defined as CMR-RF > 20%) 1 month after TAVR. Routine assessment of this readily available haemodynamic parameter should be encouraged.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Acknowledgements
None declared.
Funding
This research was funded by a research grant from Abbott Laboratories (Chicago, Illinois, USA).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: M.J.P.R., N.A.S., K.v.d.W., L.R., H.G., L.A.F.M.v.G., G.S.C.G., M.W.A.V., J.H., S.E.M., and D.H.J.T. do not have potential conflicts of interest or disclosures to report. M.H.v.W. has been a proctor and consultant for Abbott Vascular. R.N. has received research funding from Philips Volcano and Biotronik. N.v.R. has received research funding from Abbott, Philips, Medtronic, and Biotronik; has served as a consultant for RainMed, Castor, and Medtronic; and received speaker fees from Abbott and Bayer.



