-
PDF
- Split View
-
Views
-
Cite
Cite
Aleksei Zyrianov, Paolo Spirito, Raffaele Abete, Davide Margonato, Daniele Poggio, Giuseppe Vaccari, Irene Binaco, Massimiliano Grillo, Lucian Dorobantu, Luca Boni, Paolo Ferrazzi, Impact of secondary mitral valve chordal cutting on valve geometry in obstructive hypertrophic cardiomyopathy with marked septal hypertrophy, European Heart Journal - Cardiovascular Imaging, Volume 24, Issue 5, May 2023, Pages 678–686, https://doi.org/10.1093/ehjci/jeac179
Close - Share Icon Share
Abstract
In patients with obstructive hypertrophic cardiomyopathy (HCM) and mild septal thickness undergoing myectomy, resecting fibrotic anterior mitral leaflet (AML) secondary chordae moves the mitral valve (MV) away from the outflow tract and ejection flow, reducing the need for a deep septal excision. Aim of the present study was to assess whether chordal resection has similarly favourable effects in patients with important hypertrophy, who represent the majority of patients with obstructive HCM.
The MV position in the ventricular cavity, assessed from echocardiography as AML-annulus ratio, was compared before and after chordal resection in 150 consecutive HCM patients with important (≥20 mm) and 62 with mild (≤19 mm) septal thickness undergoing myectomy. Preoperatively, MV position was displaced towards the septum to a similar extent in both groups. Postoperatively, AML-annulus ratio increased of an equal degree in both groups, from 0.43 ± 0.05 to 0.55 ± 0.06 (P < 0.001) a 28% increase, and from 0.43 ± 0.06 to 0.55 ± 0.06 (P < 0.001) a 26% increase, respectively, indicating a similar MV shift away from the outflow tract. When AML-annulus ratio was compared in the study cohort and 124 normal subjects, MV position was within normal range in <4% of patients preoperatively and normalized in >50% postoperatively.
In obstructive HCM, displacement of the MV apparatus into the outflow tract interferes with the ejection flow. Resection of fibrotic secondary chordae moves the MV apparatus away from the outflow tract and enlarges the outflow area independently of septal thickness, facilitating septal myectomy by reducing the need for a deep muscular excision.
Left ventricular (LV) outflow obstruction is the most common cause of severe heart failure symptoms in hypertrophic cardiomyopathy (HCM), and transaortic extended septal myectomy is the most effective treatment to abolish the LV outflow gradient, improve quality of life, and increase survival in HCM patients with outflow obstruction.1–3 However, the heterogeneous septal morphology and complex mechanisms responsible for LV outflow obstruction in HCM require particularly large experience with septal myectomy.1–3 At present, the number of experienced surgeons and centers in the United States and Europe is insufficient to guarantee optimal surgical treatment of LV outflow obstruction to the high number of patients with HCM eligible for surgical relief of the outflow gradient.3–6
Recently, in patients with obstructive HCM and only mild septal thickness, cutting fibrotic and/or shortened secondary chordae of the anterior mitral leaflet (AML) in association with a shallow septal myectomy has been shown to move the mitral valve (MV) apparatus away from the LV outflow tract and closer to the posterior wall, enlarging the outflow tract area and reducing the need for a deep septal excision, while isolated septal myectomy did not change the MV position in the ventricular cavity in a control group with mild septal hypertrophy.7 Whether chordal resection has similarly favorable effects in patients with important hypertrophy, who represent the majority of patients with obstructive HCM, remains undetermined.
In the present study, we assessed with echocardiography the effects of chordal cutting on the position of the MV apparatus in the ventricular cavity in a large and consecutive cohort of HCM patients undergoing transaortic septal myectomy, including patients with important and patients with only mild septal thickness, the latter patients serving as a control group. As part of our investigation, we also discuss the pathophysiologic mechanisms by which resecting fibrotic and/or shortened secondary AML chordae moves the MV apparatus away from the LV outflow tract, as well as the potential implications of this novel procedure in the surgical treatment of LV outflow obstruction in HCM.
Methods
Study population
Between January 2015 and December 2018, 226 consecutive patients with obstructive HCM underwent secondary MV chordal resection in association with a relatively shallow septal myectomy at our center and were enrolled in the study. Each patient had a LV outflow gradient ≥50 mmHg at rest or with physiologic provocation and disabling heart failure symptoms unresponsive to medications. Standard echocardiographic measurements were assessed before the myectomy operation and at the most recent clinical evaluation.2 MV regurgitation was assessed using multiple Doppler echocardiographic criteria.8 This study complies with the Declaration of Helsinki and was approved by our institutional ethical committees. A written informed consent was obtained from all study patients.
Surgical procedure
Intraoperative transesophageal echocardiography (TEE) was performed to visualize the ventricular septum and assess MV morphology and associated MV abnormalities.7,9 The papillary muscles were mobilized with the technique originally described by Messmer10 and routinely used at our center.7,9 Septal muscular resection was performed as previously described.7,9 In particular, the muscle from the anterior septum was excised in a single piece in order to leave a smooth surface on the remaining septum, and great care was taken to ensure that the muscular resection went beyond the point of mitral-septal contact.7,9 In each of the 226 study patients, the thickness and length of the muscle excised from the anterior septum was measured in the operating room. On the basis of our previous experience, a muscular excision of a depth corresponding to about 30% of the anterior septum maximal thickness was considered adequate.7 Secondary chordae judged to play an important role in tethering the AML towards the LV outflow tract were resected.7 These chordae were particularly thickened, often agglutinated and inserted into a fibrotic area of the corresponding papillary muscle. After chordal resection, the AML free margin and primary chordae were carefully explored to identify leaflet tears, usually in the portion of the leaflet involved in systolic anterior movement, and/or abnormally elongated or ruptured primary chordae. If some of these features were identified, the pertinent portion of the leaflet was plicated, and/or the two chordae contiguous to the one abnormally elongated or ruptured were brought closer to each other. In patients with particularly elongated anterior or posterior leaflets, the excessive tissue at the free margin of the leaflet was plicated.
Transthoracic echocardiographic assessment of the impact of chordal cutting on the MV position in the ventricular cavity
The AML to annulus coaptation ratio, measured at the onset of systole (at MV coaptation) and calculated as the ratio between AML projection on the mitral annulus plane and annulus length, was used to compare the MV position in the LV cavity before and after surgery in patients with important and patients with mild septal thickness (Figure 1). This echocardiographic index reflects the degree of anterior displacement of the MV leaflets toward the ventricular septum7,11,12 and has been used at our center since 2014.7 MV tenting area, which measures the area enclosed between MV leaflets and annulus plane at the onset of systole (at MV coaptation) and reflects the degree of MV displacement towards the LV apex,7,13 was also compared in the two patient groups.

Echocardiographic assessment of the MV position at onset of systole (at time of MV coaptation) in a patient with obstructive HCM. The anterior leaflet-annulus ratio is calculated as the ratio between AML projection on annulus plane (X) and annulus length (A1-A2). The tenting area (TA) comprises the area between the MV leaflets and annulus plane. The electrocardiogram on the top left indicates the phase of the cardiac cycle. C-P = coaptation point.
In order to compare the degree of normalization of MV position after chordal cutting in patients with important and patients with mild septal thickness, the normal range for AML to annulus coaptation ratio was also assessed in 124 normal subjects matched for age with the study patients.
Statistical analysis
Primary analyses were aimed at describing and comparing pre-operative and post-operative clinical characteristics in the study patients, according to their degree of septal hypertrophy evaluated before myectomy. Variables were presented as means with standard deviations, or frequencies with proportions. Differences between the two groups of patients with mild (≤19 mm) and with more marked (≥20 mm) septal hypertrophy were analyzed using the Wilcoxon–Mann–Whitney test for continuous variables, while categorical variables were compared using the χ2 test for heterogeneity. Differences between pre-operative and post-operative values were assessed for statistical significance using the paired Student t-test, McNemar's test, or Bowker's Test of Symmetry. The presence of interaction between time of measurements (before and after surgery) and degree of septal hypertrophy was tested using the repeated measures analysis of variance or the alternating logistic regression model associated with the generalized estimating equations approach. The changes observed after surgery in the AML-annulus ratio and MV tenting area in the patients with mild and more marked septal hypertrophy were compared using the standard analysis of covariance, with post-operative values as the dependent variable and pre-operative values as covariates. Values of P < 0.05 were considered statistically significant. No adjustments for multiple comparisons were performed. Reference ranges for AML-annulus ratio and MV tenting area in normal subjects were computed according to the Clinical and Laboratory Standards Institute Guidelines 2010. SAS System 9.4 was used in all statistical analyses.
Results
Clinical results
Of the 226 study patients, 162 had important septal hypertrophy (≥20 mm), mean 25 ± 4, and 64 had mild septal hypertrophy (≤19 mm), mean 18 ± 2 mm, before myectomy. Of these 226 study patients, none had iatrogenic septal defect. The baselined preoperative clinical characteristics in the study patients are reported in Table 1.
Baseline clinical characteristics in patients with mild (≤ 19 mm) and patients with more marked (≥ 20 mm) septal hypertrophy
| Variables . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 162) . | P-value . |
|---|---|---|---|
| Age (years) | 56 ± 12 | 52 ± 15 | 0.055 |
| Male | 30 (47%) | 97 (60%) | 0.076 |
| NYHA functional class | 0.204 | ||
| ȃI | 0 | 0 | |
| ȃII | 10 (16%) | 38 (23%) | |
| ȃIII | 50 (78%) | 107 (66%) | |
| ȃIV | 4 (6%) | 17 (11%) | |
| Atrial fibrillation | 0.344 | ||
| ȃNo | 49 (77%) | 133 (82%) | |
| ȃYes | 15 (23%) | 29 (18%) | |
| Ecocardiographic data | |||
| LVOT gradient at rest, mmHg | 71 ± 41 | 70 ± 34 | 0.445 |
| Septal thickness, mm | 18 ± 1 | 25 ± 4 | — |
| MV regurgitation | 0.626 | ||
| ȃ0–1 | 20 (31%) | 44 (27%) | |
| ȃ2 | 25 (39%) | 72 (44%) | |
| ȃ3 | 8 (13%) | 26 (16%) | |
| ȃ4 | 11 (17%) | 20 (12%) |
| Variables . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 162) . | P-value . |
|---|---|---|---|
| Age (years) | 56 ± 12 | 52 ± 15 | 0.055 |
| Male | 30 (47%) | 97 (60%) | 0.076 |
| NYHA functional class | 0.204 | ||
| ȃI | 0 | 0 | |
| ȃII | 10 (16%) | 38 (23%) | |
| ȃIII | 50 (78%) | 107 (66%) | |
| ȃIV | 4 (6%) | 17 (11%) | |
| Atrial fibrillation | 0.344 | ||
| ȃNo | 49 (77%) | 133 (82%) | |
| ȃYes | 15 (23%) | 29 (18%) | |
| Ecocardiographic data | |||
| LVOT gradient at rest, mmHg | 71 ± 41 | 70 ± 34 | 0.445 |
| Septal thickness, mm | 18 ± 1 | 25 ± 4 | — |
| MV regurgitation | 0.626 | ||
| ȃ0–1 | 20 (31%) | 44 (27%) | |
| ȃ2 | 25 (39%) | 72 (44%) | |
| ȃ3 | 8 (13%) | 26 (16%) | |
| ȃ4 | 11 (17%) | 20 (12%) |
LVOT = left ventricular outflow tract.
Baseline clinical characteristics in patients with mild (≤ 19 mm) and patients with more marked (≥ 20 mm) septal hypertrophy
| Variables . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 162) . | P-value . |
|---|---|---|---|
| Age (years) | 56 ± 12 | 52 ± 15 | 0.055 |
| Male | 30 (47%) | 97 (60%) | 0.076 |
| NYHA functional class | 0.204 | ||
| ȃI | 0 | 0 | |
| ȃII | 10 (16%) | 38 (23%) | |
| ȃIII | 50 (78%) | 107 (66%) | |
| ȃIV | 4 (6%) | 17 (11%) | |
| Atrial fibrillation | 0.344 | ||
| ȃNo | 49 (77%) | 133 (82%) | |
| ȃYes | 15 (23%) | 29 (18%) | |
| Ecocardiographic data | |||
| LVOT gradient at rest, mmHg | 71 ± 41 | 70 ± 34 | 0.445 |
| Septal thickness, mm | 18 ± 1 | 25 ± 4 | — |
| MV regurgitation | 0.626 | ||
| ȃ0–1 | 20 (31%) | 44 (27%) | |
| ȃ2 | 25 (39%) | 72 (44%) | |
| ȃ3 | 8 (13%) | 26 (16%) | |
| ȃ4 | 11 (17%) | 20 (12%) |
| Variables . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 162) . | P-value . |
|---|---|---|---|
| Age (years) | 56 ± 12 | 52 ± 15 | 0.055 |
| Male | 30 (47%) | 97 (60%) | 0.076 |
| NYHA functional class | 0.204 | ||
| ȃI | 0 | 0 | |
| ȃII | 10 (16%) | 38 (23%) | |
| ȃIII | 50 (78%) | 107 (66%) | |
| ȃIV | 4 (6%) | 17 (11%) | |
| Atrial fibrillation | 0.344 | ||
| ȃNo | 49 (77%) | 133 (82%) | |
| ȃYes | 15 (23%) | 29 (18%) | |
| Ecocardiographic data | |||
| LVOT gradient at rest, mmHg | 71 ± 41 | 70 ± 34 | 0.445 |
| Septal thickness, mm | 18 ± 1 | 25 ± 4 | — |
| MV regurgitation | 0.626 | ||
| ȃ0–1 | 20 (31%) | 44 (27%) | |
| ȃ2 | 25 (39%) | 72 (44%) | |
| ȃ3 | 8 (13%) | 26 (16%) | |
| ȃ4 | 11 (17%) | 20 (12%) |
LVOT = left ventricular outflow tract.
Of the 226 study patients, one died of sepsis during hospitalization 60 days after surgery (perioperative mortality 0.4%), none had iatrogenic septal defect. Postoperatively, 3 (2%) patients with important and none of those with mild septal hypertrophy had a resting peak LV outflow gradient ≥30 mmHg; 2 (1%) of the patients with important septal hypertrophy and none of those with mild septal hypertrophy had residual significant heart failure symptoms (NYHA class III). Both patient groups showed a significant and similar postoperative increase in end-systolic volume, without a parallel increase in end-diastolic volume and a consequent modest decrease in LV ejection fraction. The pre and postoperative clinical characteristics in the two patient groups are reported in Table 2.
| Parameter . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 161)* . | P-value for interaction . | ||||
|---|---|---|---|---|---|---|---|
| . | Before surgery . | After surgery . | P-value . | Before surgery . | After surgery . | P-value . | . |
| NYHA functional class | |||||||
| ȃI | 0 | 56 (87%) | <0.001 | 0 | 132 (82%) | <0.001 | 0.202 |
| ȃII | 10 (16%) | ȃ8 (13%) | ȃ38 (24%) | ȃ27 (17%) | |||
| ȃIII | 50 (78%) | 0 | 106 (66%) | ȃ2 (1%) | |||
| ȃIV | 4 (6%) | 0 | ȃ17 (11%) | 0 | |||
| Atrial fibrillation | |||||||
| ȃNo | 49 (77%) | 59 (92%) | 0.004 | 133 (83%) | 151 (94%) | <0.001 | 0.812 |
| ȃYes | 15 (23%) | 5 (8%) | ȃ28 (17%) | 10 (6%) | |||
| Ecocardiographic data | |||||||
| LVOT gradient at rest, mmHg | 71 ± 41 | 11 ± 5 | <0.001 | 69 ± 34 | 11 ± 6 | <0.001 | 0.813 |
| Septal thickness, mm | 18 ± 2 | 14 ± 2 | <0.001 | 25 ± 4 | 17 ± 4 | <0.001 | <0.001 |
| Post-op gradient at rest ≥30 mmHg | |||||||
| ȃNo | ȃNA | ȃ64 (100%) | NA | ȃNA | ȃ158 (98%) | NA | NA |
| ȃYes | ȃNA | 0 | ȃNA | ȃ3 (2%) | |||
| LV ejection fraction, % | 67 ± 6 | 63 ± 6 | <0.001 | 65 ± 6 | 63 ± 6 | 0.003 | 0.135 |
| LV end systolic volume, mL | ȃ28 ± 11 | 33 ± 12 | 0.003 | 33 ± 14 | 38 ± 15 | <0.001 | 0.627 |
| LV end diastolic volume, mL | ȃ88 ± 29 | 89 ± 23 | 0.860 | 95 ± 30 | 102 ± 29 | 0.013 | 0.224 |
| MV regurgitation | |||||||
| ȃ0–1 | 20 (31%) | 55 (86%) | <0.001 | 44 (27%) | 139 (86%) | <0.001 | 0.911 |
| ȃ2 | 25 (39%) | ȃ8 (13%) | 71 (44%) | ȃ19 (12%) | |||
| ȃ3 | ȃ8 (13%) | 1 (2%) | 26 (16%) | ȃ3 (2%) | |||
| ȃ4 | 11 (17%) | ȃ0 | 20 (12%) | ȃ0 | |||
| Parameter . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 161)* . | P-value for interaction . | ||||
|---|---|---|---|---|---|---|---|
| . | Before surgery . | After surgery . | P-value . | Before surgery . | After surgery . | P-value . | . |
| NYHA functional class | |||||||
| ȃI | 0 | 56 (87%) | <0.001 | 0 | 132 (82%) | <0.001 | 0.202 |
| ȃII | 10 (16%) | ȃ8 (13%) | ȃ38 (24%) | ȃ27 (17%) | |||
| ȃIII | 50 (78%) | 0 | 106 (66%) | ȃ2 (1%) | |||
| ȃIV | 4 (6%) | 0 | ȃ17 (11%) | 0 | |||
| Atrial fibrillation | |||||||
| ȃNo | 49 (77%) | 59 (92%) | 0.004 | 133 (83%) | 151 (94%) | <0.001 | 0.812 |
| ȃYes | 15 (23%) | 5 (8%) | ȃ28 (17%) | 10 (6%) | |||
| Ecocardiographic data | |||||||
| LVOT gradient at rest, mmHg | 71 ± 41 | 11 ± 5 | <0.001 | 69 ± 34 | 11 ± 6 | <0.001 | 0.813 |
| Septal thickness, mm | 18 ± 2 | 14 ± 2 | <0.001 | 25 ± 4 | 17 ± 4 | <0.001 | <0.001 |
| Post-op gradient at rest ≥30 mmHg | |||||||
| ȃNo | ȃNA | ȃ64 (100%) | NA | ȃNA | ȃ158 (98%) | NA | NA |
| ȃYes | ȃNA | 0 | ȃNA | ȃ3 (2%) | |||
| LV ejection fraction, % | 67 ± 6 | 63 ± 6 | <0.001 | 65 ± 6 | 63 ± 6 | 0.003 | 0.135 |
| LV end systolic volume, mL | ȃ28 ± 11 | 33 ± 12 | 0.003 | 33 ± 14 | 38 ± 15 | <0.001 | 0.627 |
| LV end diastolic volume, mL | ȃ88 ± 29 | 89 ± 23 | 0.860 | 95 ± 30 | 102 ± 29 | 0.013 | 0.224 |
| MV regurgitation | |||||||
| ȃ0–1 | 20 (31%) | 55 (86%) | <0.001 | 44 (27%) | 139 (86%) | <0.001 | 0.911 |
| ȃ2 | 25 (39%) | ȃ8 (13%) | 71 (44%) | ȃ19 (12%) | |||
| ȃ3 | ȃ8 (13%) | 1 (2%) | 26 (16%) | ȃ3 (2%) | |||
| ȃ4 | 11 (17%) | ȃ0 | 20 (12%) | ȃ0 | |||
LV = left ventricle; LVOT = left ventricular outflow tract; MV = mitral valve; NA = not applicable; NYHA = New York Heart Association.
Symbols: *One of the 226 study patients died postoperatively during hospitalization.
| Parameter . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 161)* . | P-value for interaction . | ||||
|---|---|---|---|---|---|---|---|
| . | Before surgery . | After surgery . | P-value . | Before surgery . | After surgery . | P-value . | . |
| NYHA functional class | |||||||
| ȃI | 0 | 56 (87%) | <0.001 | 0 | 132 (82%) | <0.001 | 0.202 |
| ȃII | 10 (16%) | ȃ8 (13%) | ȃ38 (24%) | ȃ27 (17%) | |||
| ȃIII | 50 (78%) | 0 | 106 (66%) | ȃ2 (1%) | |||
| ȃIV | 4 (6%) | 0 | ȃ17 (11%) | 0 | |||
| Atrial fibrillation | |||||||
| ȃNo | 49 (77%) | 59 (92%) | 0.004 | 133 (83%) | 151 (94%) | <0.001 | 0.812 |
| ȃYes | 15 (23%) | 5 (8%) | ȃ28 (17%) | 10 (6%) | |||
| Ecocardiographic data | |||||||
| LVOT gradient at rest, mmHg | 71 ± 41 | 11 ± 5 | <0.001 | 69 ± 34 | 11 ± 6 | <0.001 | 0.813 |
| Septal thickness, mm | 18 ± 2 | 14 ± 2 | <0.001 | 25 ± 4 | 17 ± 4 | <0.001 | <0.001 |
| Post-op gradient at rest ≥30 mmHg | |||||||
| ȃNo | ȃNA | ȃ64 (100%) | NA | ȃNA | ȃ158 (98%) | NA | NA |
| ȃYes | ȃNA | 0 | ȃNA | ȃ3 (2%) | |||
| LV ejection fraction, % | 67 ± 6 | 63 ± 6 | <0.001 | 65 ± 6 | 63 ± 6 | 0.003 | 0.135 |
| LV end systolic volume, mL | ȃ28 ± 11 | 33 ± 12 | 0.003 | 33 ± 14 | 38 ± 15 | <0.001 | 0.627 |
| LV end diastolic volume, mL | ȃ88 ± 29 | 89 ± 23 | 0.860 | 95 ± 30 | 102 ± 29 | 0.013 | 0.224 |
| MV regurgitation | |||||||
| ȃ0–1 | 20 (31%) | 55 (86%) | <0.001 | 44 (27%) | 139 (86%) | <0.001 | 0.911 |
| ȃ2 | 25 (39%) | ȃ8 (13%) | 71 (44%) | ȃ19 (12%) | |||
| ȃ3 | ȃ8 (13%) | 1 (2%) | 26 (16%) | ȃ3 (2%) | |||
| ȃ4 | 11 (17%) | ȃ0 | 20 (12%) | ȃ0 | |||
| Parameter . | Maximal septal thickness ≤19 mm (N = 64) . | Maximal septal thickness ≥20 mm (N = 161)* . | P-value for interaction . | ||||
|---|---|---|---|---|---|---|---|
| . | Before surgery . | After surgery . | P-value . | Before surgery . | After surgery . | P-value . | . |
| NYHA functional class | |||||||
| ȃI | 0 | 56 (87%) | <0.001 | 0 | 132 (82%) | <0.001 | 0.202 |
| ȃII | 10 (16%) | ȃ8 (13%) | ȃ38 (24%) | ȃ27 (17%) | |||
| ȃIII | 50 (78%) | 0 | 106 (66%) | ȃ2 (1%) | |||
| ȃIV | 4 (6%) | 0 | ȃ17 (11%) | 0 | |||
| Atrial fibrillation | |||||||
| ȃNo | 49 (77%) | 59 (92%) | 0.004 | 133 (83%) | 151 (94%) | <0.001 | 0.812 |
| ȃYes | 15 (23%) | 5 (8%) | ȃ28 (17%) | 10 (6%) | |||
| Ecocardiographic data | |||||||
| LVOT gradient at rest, mmHg | 71 ± 41 | 11 ± 5 | <0.001 | 69 ± 34 | 11 ± 6 | <0.001 | 0.813 |
| Septal thickness, mm | 18 ± 2 | 14 ± 2 | <0.001 | 25 ± 4 | 17 ± 4 | <0.001 | <0.001 |
| Post-op gradient at rest ≥30 mmHg | |||||||
| ȃNo | ȃNA | ȃ64 (100%) | NA | ȃNA | ȃ158 (98%) | NA | NA |
| ȃYes | ȃNA | 0 | ȃNA | ȃ3 (2%) | |||
| LV ejection fraction, % | 67 ± 6 | 63 ± 6 | <0.001 | 65 ± 6 | 63 ± 6 | 0.003 | 0.135 |
| LV end systolic volume, mL | ȃ28 ± 11 | 33 ± 12 | 0.003 | 33 ± 14 | 38 ± 15 | <0.001 | 0.627 |
| LV end diastolic volume, mL | ȃ88 ± 29 | 89 ± 23 | 0.860 | 95 ± 30 | 102 ± 29 | 0.013 | 0.224 |
| MV regurgitation | |||||||
| ȃ0–1 | 20 (31%) | 55 (86%) | <0.001 | 44 (27%) | 139 (86%) | <0.001 | 0.911 |
| ȃ2 | 25 (39%) | ȃ8 (13%) | 71 (44%) | ȃ19 (12%) | |||
| ȃ3 | ȃ8 (13%) | 1 (2%) | 26 (16%) | ȃ3 (2%) | |||
| ȃ4 | 11 (17%) | ȃ0 | 20 (12%) | ȃ0 | |||
LV = left ventricle; LVOT = left ventricular outflow tract; MV = mitral valve; NA = not applicable; NYHA = New York Heart Association.
Symbols: *One of the 226 study patients died postoperatively during hospitalization.
At transthoracic echocardiography, maximal septal thickness decreased from 25 ± 4 mm preoperatively to 17 ± 4 mm postoperatively (minus 32%) in patients with important septal hypertrophy and from 18 ± 2 mm preoperatively to 14 ± 2 mm postoperatively (minus 22%) in those with mild septal hypertrophy. Thickness and length of the excised muscle measured in the operating room were 9 ± 3 mm and 41 + 9 mm, respectively, in patients with important hypertrophy, and 7 ± 2 mm and 36 ± 9 mm, respectively, in those with mild septal hypertrophy. Images of excised septal muscle and chordae in a patient with marked and one with mild septal thickness are shown in Figures 2 and 3.
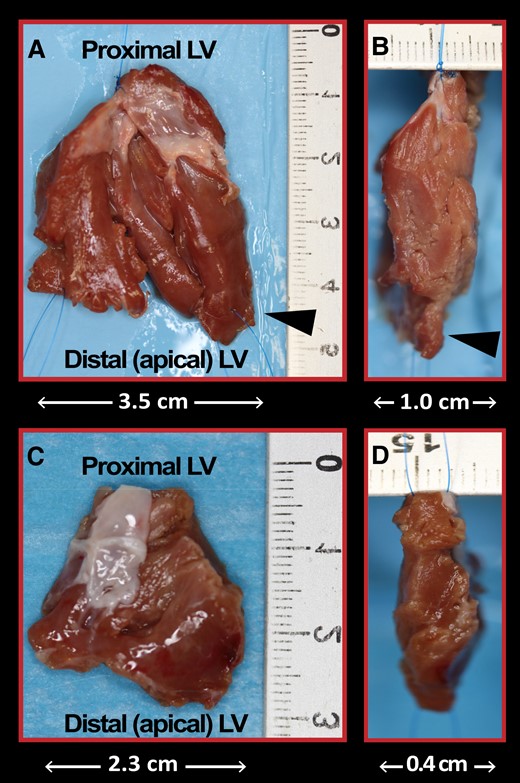
Resected septal muscle at myectomy. Antero-posterior and lateral views of the resected muscle from the anterior ventricular septum in a patient with obstructive HCM and particularly marked septal hypertrophy (35 mm) (A and B) and one with mild septal hypertrophy (16 mm) (C and D). The cardiac muscle was resected via two incisions in the basal septum 2 to 3 mm below the aortic valve and extended distally to the base of the papillary muscles. Anomalous muscle bundles that contributed to reduce the size of the midventricular cavity were also resected (arrowheads).
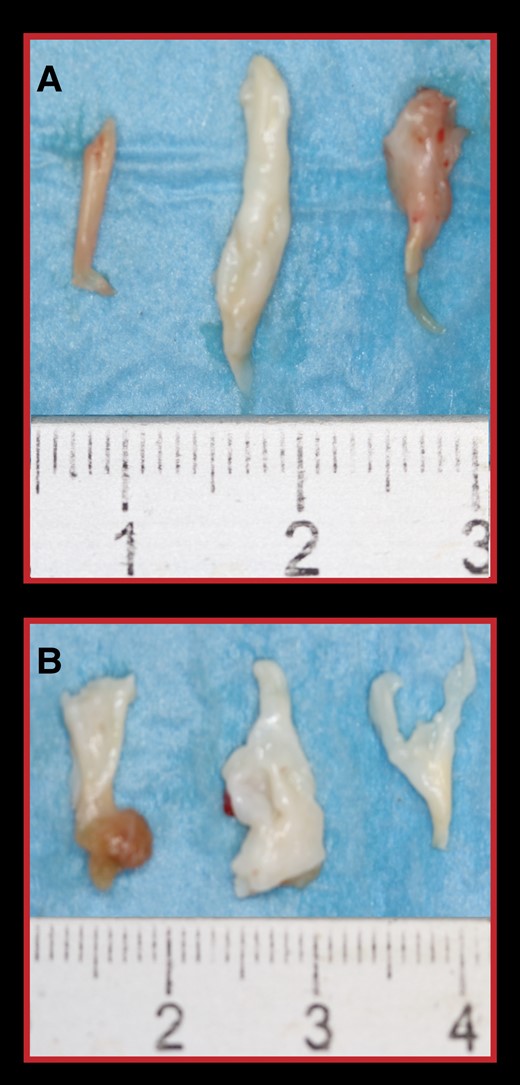
Resected fibrotic and retracted secondary chordae of the anterior MV leaflet in the two patients showed in Figure 2. (A) Secondary chordae from the patient A–B. (B) Secondary chordae from the patient C–D.
MV position in the ventricular cavity before and after surgery
Because of the insufficient quality of the transthoracic echocardiogram, 14 study patients were excluded from the assessment of MV position in the cavity. One patient with important septal hypertrophy died during hospitalization. MV position was assessed in the remaining 150 patients with important and 62 with mild septal hypertrophy.
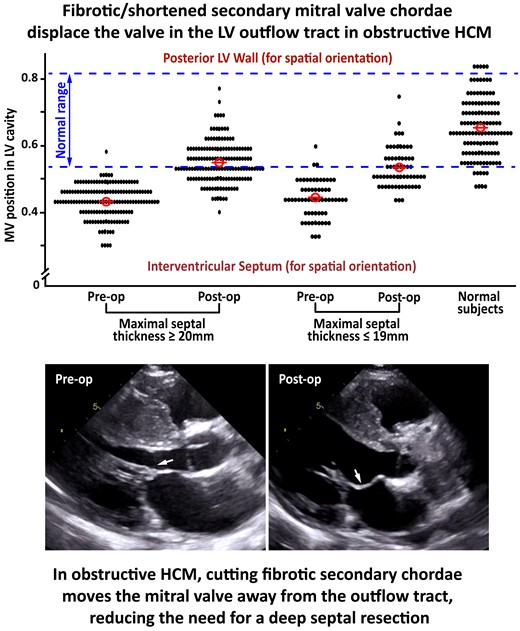
Preoperatively, the AML to annulus coaptation ratio was 0.43 ± 0.05 (median 0.44) in patients with important septal hypertrophy and 0.43 ± 0.06 (median 0.43) in those with mild septal hypertrophy, indicating that at onset of systole the MV apparatus was displaced towards the LV outflow tract to an equal extent in the two groups, independently of septal thickness. This index increased from 0.43 ± 0.05 preoperatively to 0.55 ± 0.06 postoperatively (P < 0.001), a 28% increase in patients with important septal hypertrophy, and from 0.43 ± 0.06 to 0.55 ± 0.06 (P < 0.001), a 26% increase, in those with mild hypertrophy, showing a similar shift towards normalization of MV position in the two groups, closer to the posterior LV wall and away from the outflow tract, with a consequent increase in LV outflow tract dimension (Table 3) (Figures 4 to 6).
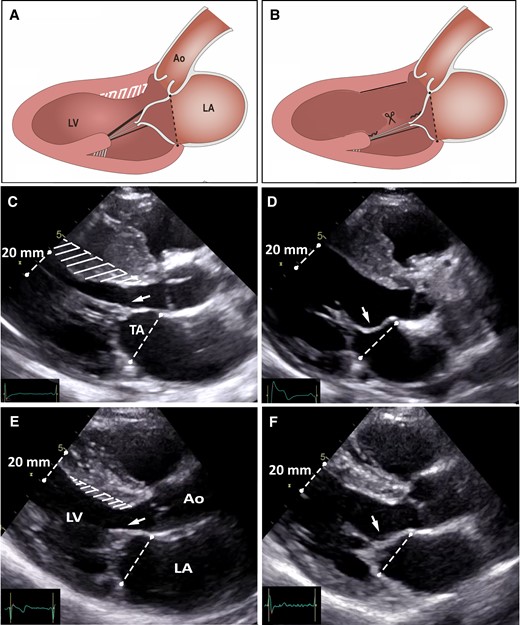
Illustration of the operative results. (A and B) Schematic representation of the traction exerted by fibrotic secondary MV chordae on the AML, and the results of resecting fibrotic secondary chordae (combined with a shallow myectomy) on the MV apparatus. (C and D) Pre-operative and post-operative echocardiographic images obtained in early systole in a patient with obstructive HCM and marked septal thickness, and (E and F) in a patient with obstructive HCM and mild septal thickness. Preoperatively, in both patients, fibrotic and retracted secondary AML chordae cause abnormal tethering of the AML with displacement of the MV valve apparatus towards the LV outflow tract (arrows). Postoperatively, the AML tethering is abolished (arrows), the MV apparatus has moved away from the LV outflow tract to a more posterior and normal position in the LV cavity. The MV tenting area is also substantially reduced.
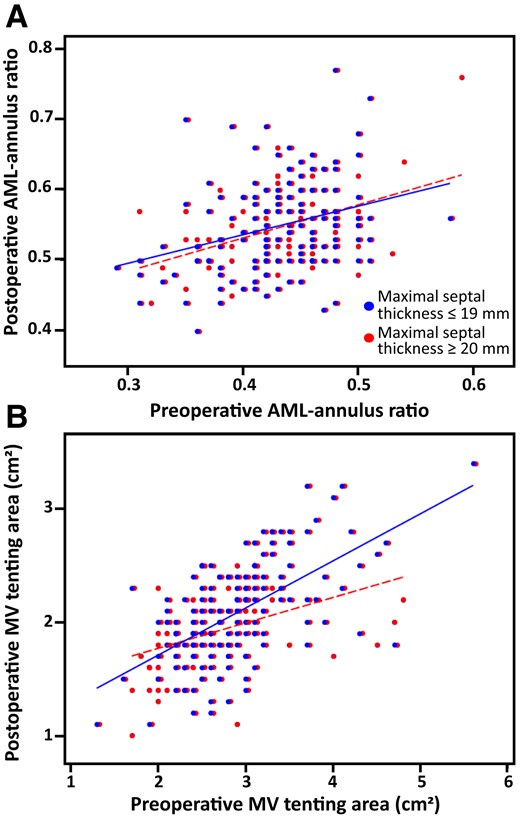
Scatterplots showing preoperative and postoperative echocardiographic measurements of the AML-annulus ratio and tenting area in patients with important (≥ 20 mm) and patients with mild (≤ 19 mm) septal hypertrophy. (A) AML-annulus ratio postoperative values are, on average, superimposable in the two study groups, and systematically higher than the preoperative values in the great majority of patients. (B) Tenting area postoperative values are, on average, superimposable in the two study groups, and systematically lower than the preoperative values in the great majority of patients.
| . | Maximal septal thickness ≤19 mm (mean ± SD) . | Maximal septal thickness ≥20 mm (mean ± SD) . | Crude post-operative difference between groups (mean ± SE) . | Adjusted post-operative difference between groups (mean ± SE) . | P value . |
|---|---|---|---|---|---|
| AML-annulus ratio | |||||
| ȃPre-operative value | 0.43 ± 0.06 | 0.43 ± 0.05 | |||
| ȃPost-operative value | 0.55 ± 0.06 | 0.55 ± 0.06 | –0.002 ± 0.01 | –0.002 ± 0.01 | 0.780 |
| ȃAbsolute change | +0.11 ± 0.06 | +0.12 ± 0.07 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | +26% | +28% | NM | ||
| MV tenting area, cm2 | |||||
| ȃPre-operative value | 2.65 ± 0.67 | 2.89 ± 0.64 | |||
| ȃPost-operative value | 1.91 ± 0.36 | 2.08 ± 0.44 | –0.16 ± 0.06 | –0.08 ± 0.05 | 0.150 |
| ȃAbsolute change | –0.73 ± 0.61 | –0.81 ± 0.52 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | –28% | –28% | +33% |
| . | Maximal septal thickness ≤19 mm (mean ± SD) . | Maximal septal thickness ≥20 mm (mean ± SD) . | Crude post-operative difference between groups (mean ± SE) . | Adjusted post-operative difference between groups (mean ± SE) . | P value . |
|---|---|---|---|---|---|
| AML-annulus ratio | |||||
| ȃPre-operative value | 0.43 ± 0.06 | 0.43 ± 0.05 | |||
| ȃPost-operative value | 0.55 ± 0.06 | 0.55 ± 0.06 | –0.002 ± 0.01 | –0.002 ± 0.01 | 0.780 |
| ȃAbsolute change | +0.11 ± 0.06 | +0.12 ± 0.07 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | +26% | +28% | NM | ||
| MV tenting area, cm2 | |||||
| ȃPre-operative value | 2.65 ± 0.67 | 2.89 ± 0.64 | |||
| ȃPost-operative value | 1.91 ± 0.36 | 2.08 ± 0.44 | –0.16 ± 0.06 | –0.08 ± 0.05 | 0.150 |
| ȃAbsolute change | –0.73 ± 0.61 | –0.81 ± 0.52 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | –28% | –28% | +33% |
NM = not measurable.
| . | Maximal septal thickness ≤19 mm (mean ± SD) . | Maximal septal thickness ≥20 mm (mean ± SD) . | Crude post-operative difference between groups (mean ± SE) . | Adjusted post-operative difference between groups (mean ± SE) . | P value . |
|---|---|---|---|---|---|
| AML-annulus ratio | |||||
| ȃPre-operative value | 0.43 ± 0.06 | 0.43 ± 0.05 | |||
| ȃPost-operative value | 0.55 ± 0.06 | 0.55 ± 0.06 | –0.002 ± 0.01 | –0.002 ± 0.01 | 0.780 |
| ȃAbsolute change | +0.11 ± 0.06 | +0.12 ± 0.07 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | +26% | +28% | NM | ||
| MV tenting area, cm2 | |||||
| ȃPre-operative value | 2.65 ± 0.67 | 2.89 ± 0.64 | |||
| ȃPost-operative value | 1.91 ± 0.36 | 2.08 ± 0.44 | –0.16 ± 0.06 | –0.08 ± 0.05 | 0.150 |
| ȃAbsolute change | –0.73 ± 0.61 | –0.81 ± 0.52 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | –28% | –28% | +33% |
| . | Maximal septal thickness ≤19 mm (mean ± SD) . | Maximal septal thickness ≥20 mm (mean ± SD) . | Crude post-operative difference between groups (mean ± SE) . | Adjusted post-operative difference between groups (mean ± SE) . | P value . |
|---|---|---|---|---|---|
| AML-annulus ratio | |||||
| ȃPre-operative value | 0.43 ± 0.06 | 0.43 ± 0.05 | |||
| ȃPost-operative value | 0.55 ± 0.06 | 0.55 ± 0.06 | –0.002 ± 0.01 | –0.002 ± 0.01 | 0.780 |
| ȃAbsolute change | +0.11 ± 0.06 | +0.12 ± 0.07 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | +26% | +28% | NM | ||
| MV tenting area, cm2 | |||||
| ȃPre-operative value | 2.65 ± 0.67 | 2.89 ± 0.64 | |||
| ȃPost-operative value | 1.91 ± 0.36 | 2.08 ± 0.44 | –0.16 ± 0.06 | –0.08 ± 0.05 | 0.150 |
| ȃAbsolute change | –0.73 ± 0.61 | –0.81 ± 0.52 | |||
| ȃP value | <0.001 | <0.001 | |||
| ȃProportional change | –28% | –28% | +33% |
NM = not measurable.
MV tenting area decreased from 2.89 ± 0.64 preoperatively to 2.08 ± 0.44 cm2 postoperatively (P < 0.001), a 28% decrease in patients with important hypertrophy, and from 2.65 ± 0.67 cm2 (median 2.8 cm2) to 1.91 ± 0.36 cm2 (P < 0.001), a 28% decrease, in those with mild hypertrophy, showing a similar shift towards normalization of MV position in the two groups, away from the apex and closer to the MV annulus plane (Table 3) (Figures 4 and 6).
The degree of normalization of MV position in the ventricular cavity after surgery was quantified by comparison with the normal range for the MV position in the cavity derived from 124 normal subjects. In normal subjects, at the onset of systole, the reference range for normal values was ≥ 0.54 and ≤ 0.82 for AML-annulus ratio, and ≥ 0.90 and ≤ 2.20 for MV tenting area. When compared to normal values, preoperatively, the AML to annulus coaptation ratio was within normal limits in 1 (0.7%) of the 150 patients with important and 2 (3.2%) of the 62 patients with mild septal hypertrophy. Postoperatively, the AML to annulus coaptation ratio was within normal range in 79 (53%) of patients with important and 30 (48%) of those with mild hypertrophy (Figure 5). The increase in the proportion of patients with normal AML-annulus ratio after surgery was similar in the two groups (P = 0.150).
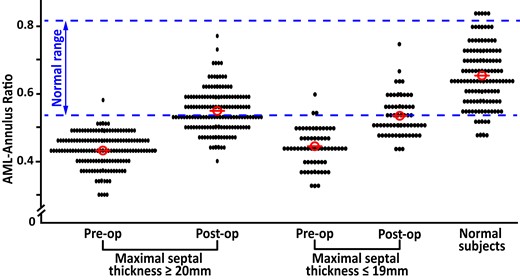
MV position at onset of systole, assessed before and after surgery as AML-annulus ratio. AML secondary chordae were resected in 150 patients with important and 62 patients with mild septal hypertrophy in association with a relatively shallow septal myectomy. The MV position in a control group of normal subjects is also shown.
Preoperatively, tenting area was within normal range in 20 (13%) of patients with important and 20 (32%) of those with mild septal hypertrophy. Postoperatively, tenting area was within normal range in 100 (67%) of patients with important and 52 (84%) of those with mild hypertrophy. The increase in the proportion of patients with a normal tenting area after surgery was similar in the two groups (P = 0.711).
Discussion
Our study shows for the first time that the traction exerted by thickened and/or shortened secondary MV chordae on the AML during systole displaces the MV apparatus into the LV outflow tract in the great majority of patients with obstructive HCM, independently of the magnitude of septal thickness, thus contributing to LV outflow obstruction. Therefore, these abnormal MV chordae play a relevant role in outflow obstruction in HCM.
Cutting these thickened secondary MV chordae (combined with a shallow septal myectomy) was previously shown to move the MV apparatus away from the LV outflow tract and the ejection flow in patients with mild septal hypertrophy.7 Whether chordal cutting has similarly favorable effects in patients with important septal hypertrophy remained undetermined.
In the present study, after resection of the thickened and/or shortened secondary MV chordae combined with a relatively shallow myectomy in a large consecutive cohort of patients with obstructive HCM, MV displacement towards the LV outflow tract decreased significantly and to a similar extent in patients with important and those with mild septal hypertrophy. The preoperative MV displacement towards the apex, assessed as MV tenting area,7,13 also decreased significantly and to a similar extent in the two patient groups. When compared to the MV position in the LV cavity assessed in a group of age-matched normal subjects, preoperatively the position of the MV apparatus was abnormal in >95% of our study patients and postoperatively moved to a normal or almost normal position in >90% of patients.
In patients with obstructive HCM, the papillary muscles are often displaced anteriorly towards the ventricular septum14–16 and the MV leaflets are elongated by progressive adaptation to the altered LV morphology17–21 or congenital persistence of muscular mitral-aortic discontinuity.22 These structural abnormalities of the MV apparatus favor systolic displacement of the AML (or both leaflets) into the LV outflow tract with consequent obstruction to the ejection flow.14–20 However, the international literature had previously ignored the role played by fibrotic and shortened MV secondary chordae on LV outflow obstruction in HCM.
The results of the present study further advance our understanding of the complex mechanisms responsible for displacement of the MV apparatus into the LV outflow area during ejection, as well as the advantages of resecting selected secondary MV chordae in patients with obstructive HCM undergoing septal myectomy. The thickened and shortened secondary chordae by exerting traction on the AML during systole, displace the MV apparatus anteriorly towards the septum and into the LV outflow tract. In addition, by reducing the tension of the primary chordae, these abnormal secondary chordae favor displacement of slack primary chordae and the contiguous AML free margin into the outflow tract by blood-flow drag forces. By resecting the anomalous secondary MV chordae, MV coaptation is moved to a more normal position in the LV inflow area contributing, in association with a relatively shallow septal myectomy, to enlarge the LV outflow tract and abolish the obstruction to the ejection flow. In addition, by abolishing the slack of the primary chordae, resection of the abnormal secondary MV chordae restores the slacked primary chordae to their function of preventing MV regurgitation.
The results of the present investigation may also have important implications for the worldwide challenge posed by the need to offer adequate surgical treatment of LV outflow obstruction to those many HCM patients eligible for transaortic septal myectomy. The small number of HCM referral centers in North America and Europe with large experience with septal myectomy continues to represent a major impediment to guarantee the gold standard treatment of LV outflow obstruction to most HCM patients with important symptoms and severely limited quality of life.3–6 The risk of iatrogenic septal defect associated with an isolated deep septal myectomy is one of the main reasons that has discouraged many experienced cardiovascular surgeons from establishing myectomy programs with the potential of offering a safe and effective treatment of LV outflow obstruction to a larger number of HCM candidates.4–6,21 The relatively shallow septal myectomy required in association with secondary AML chordal cutting, by diminishing the risk of iatrogenic septal defect, may contribute to expand the interest of cardiovascular surgeons for that large cohort of patients with obstructive HCM in search of specialized centers that can offer a surgical treatment associated with long lasting symptomatic benefit and extended longevity.
Limitations
A postoperative stress echocardiogram was not performed routinely after surgery in our study population. We considered this test unnecessary because after our operative procedure 85% of our patients were in NYHA functional class I, 2% were in class III and only 1% with a residual significant resting outflow gradient (≥ 30 mm Hg). A postoperative stress echocardiogram was performed when clinically justified. Therefore, we cannot determine the precise number of patients with a postoperative provocable LV outflow gradient in our study population.
Conclusions
Preoperatively, MV leaflets coaptation was displaced towards the LV outflow tract to an equal extent in our study patients with obstructive HCM and moderate or marked septal hypertrophy as in those with mild septal hypertrophy. Transaortic resection of abnormally thickened and/shorted secondary AML chordae moved the MV apparatus away from the LV outflow tract in the great majority of our study patients, independently of septal thickness, enlarging the outflow tract size, restoring the MV leaflets to a normal or almost normal posterior position in the ventricular cavity, contributing to the abolition of the LV outflow gradient and facilitating septal myectomy by reducing the need for a deep muscular excision.
Acknowledgements
We gratefully acknowledge the assistance of Giovanni Passafaro in the acquisition of data.
Funding
None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Author notes
Conflict of interest: None declared.



