-
PDF
- Split View
-
Views
-
Cite
Cite
J Abecasis, N Cardim, Longitudinal strain: on its way to be included in the sudden cardiac death risk models of hypertrophic cardiomyopathy?, European Heart Journal - Cardiovascular Imaging, Volume 23, Issue 8, August 2022, Pages 1016–1017, https://doi.org/10.1093/ehjci/jeac052
Close - Share Icon Share
This editorial refers to ‘Reduced myocardial septal function assessed by cardiac magnetic resonance feature tracking in patients with hypertrophic obstructive cardiomyopathy: associated with histological myocardial fibrosis and ventricular arrhythmias’, by Y. Song et al., https://doi.org/10.1093/ehjci/jeac032.
In the recent past, one of the major clinical achievements in patients with hypertrophic cardiomyopathy (HCM) was the reduction of mortality (up to the best of 0.5%/year). The dissemination of advanced heart failure therapy (including cardiac transplant), appropriate anticoagulation in atrial fibrillation, and remarkably, the use of implantable cardioverter-defibrillators (ICDs) to prevent sudden cardiac death (SCD) are important determinants of these good news for HCM patients.1
However, the optimal risk stratification strategy for ICD implantation is the context of primary prevention of SCD is an ongoing matter of debate, as distinct models have suboptimal discriminative power, particularly for distinct time frames of follow-up. In this way, individual risk should be periodically re-assessed2 and the different existing algorithms should be refined with new and better risk markers.
Novel technological advances in cardiac imaging, such as those provided by cardiac magnetic resonance (CMR), may shed a glimpse towards our understanding of the clinical impact of myocyte disarray, microvascular abnormalities, and interstitial and replacement fibrosis. This is the case of the use of late gadolinium enhancement (LGE), as its relationship with myocardial collagen histological content, a major component of replacement myocardial fibrosis (MF), was previously clearly demonstrated. LGE is now used as an independent predictor of outcomes in HCM patients, with incremental discriminatory power when added to established risk models.3
On the other hand, it has also been reported that LGE is significantly correlated with global and regional left ventricular (LV) longitudinal strain, assessed by speckle tracking echocardiography (STE). Moreover, myocardial strain seems to be affected by the global fibrotic burden (not only by replacement fibrosis), and from the magnitude of LV hypertrophy and mass.4
In the article from Song et al.5 the authors explored, in 123 patients with obstructive HCM (HOCM) before surgical myectomy, (i) if myocardial strain assessed by CMR feature tracking (CMR-FT) was related to histological MF and to the pre-operative occurrence of ventricular arrhythmia (VA), and (ii) if deformation analysis provided incremental value for indicating the presence of MF and complex VAs. HOCM patients underwent preoperative Holter monitoring and CMR-LGE-FT. Both basal anteroseptal and mid anteroseptal LGE amount and deformation curves were compared with histopathological data and with MF quantification at myectomy specimens. Among HOCM patients without LGE (51.2%), septal deformation indexes were significantly impaired in patients with increased MF (MF+). In multivariate analysis, septal longitudinal strain was an independent predictor for the presence of MF and VA. Furthermore, this variable also showed incremental value over the European Society of Cardiology (ESC) 5-year SCD risk score and over the septal LGE percentage, providing additional clues to the prediction of SCD risk in HOCM.
Though it might be argued that intraventricular obstruction and septal thickness may significantly interfere with local myocardial mechanics all patients had severe obstruction, and longitudinal strain was an independent predictor of MF at multivariate analysis. Accordingly, deformation impairment was only related to intramyocardial fibrosis, as subendocardial Masson’s Trichrome positivity, a mostly non-specific finding, was excluded from the quantification.
A previous study from Edvardsen et al.6 with a similar protocol (but with STE for strain analysis), found that septal longitudinal strain correlated with both total and interstitial MF at histology, being an independent predictor of both MF and VA occurrence. Notably, neither longitudinal strain was related to the presence of replacement fibrosis, nor LGE correlated with the total fibrotic content. This goes in line with the current study results:
Impaired longitudinal strain by CMR-FT, reflecting subendocardial function, is a sensitive marker of cardiac events, specifically of VA, and MF underlies this relationship. Disarray, diastolic impairment, coronary microvascular dysfunction, and ischaemia may be the drivers.7
MF occurs in myocardial segments with and without LGE, and its amount is not significantly different in both groups. As previously described, the histological basis of LGE is complex and non-specific in HCM, and its correlation with ‘strict’ MF is not perfect. In fact, LGE may represent other types of fibrosis, besides replacement fibrosis: plexiform fibrosis associated with disarray, perivascular fibrosis, and microscopic replacement scars.8 Furthermore, LGE also relies on an imaging cut-off measurement (‘all or none’), so that we are measuring different tissue components with different tools as far as fibrosis, myocardial histology, and LGE are concerned. That said, the histological distinction between interstitial, perivascular and replacement fibrosis would have been very useful in this study.
In the same scope, parametric CMR tools, such as T1 mapping9,10 and extracellular volume estimation, would have enlightened the study, not only for correlation with MF and collagen volume fraction but also for incremental risk assessment. As in other cardiomyopathies, the optimal non-invasive MF measurement technique, where ECV is included, will facilitate biological insights, support risk stratification, and even antifibrotic therapies for HCM patients, holding promise for future precision medicine.
For all that was mentioned it is easy to understand why longitudinal strain, as an independent predictor of MF, has incremental value over the ESC-SCD score. The presence of diffuse interstitial fibrosis may also favour the onset of re-entrant activity from non-conducting collagen septa between myocytes. On the other hand, LGE-associated arrhythmic risk may be gathered from several, distinct but concomitant and inter-related pathophysiologic processes leading to structural sequelae with longer-term prognostic impact.
Overall, when tissue characterization by CMR is in the spotlight, LGE is important, but still one of the pieces of a broadly complex puzzle not completely understood. In the case of longitudinal strain assessment in HCM, regional deformation impairment may stem from the complex interaction between interstitial fibrosis, extracellular matrix remodelling, cardiac fibres disarray, and small vessel disease, evaluating the impact of underlying pathology on regional function. As brought from this article, myocardial strain may be an ultrastructural change surrogate marker. Even so, it may provide clinical correlation data with risk stratification improvement, probably because deformation is the result of complex interactions, where the degree of fibrosis is no less than important (Figure 1).
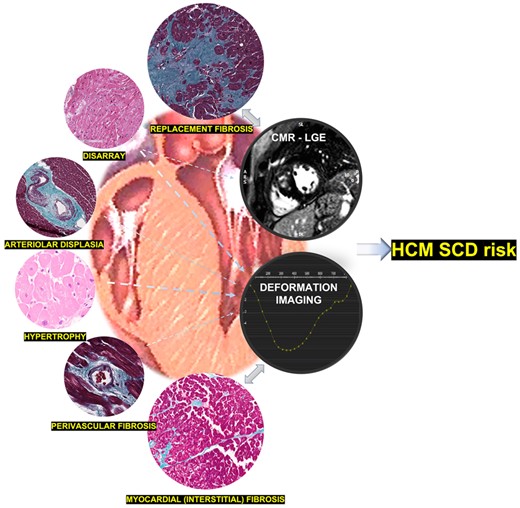
HCM-SCD risk scores should incorporate additional imaging markers (such as longitudinal strain and LGE analysis), based on distinct ultrastructural changes.
Once more, a one-stop shot comprehensive CMR-LGE-FT study stands for a whole-heart non-invasive biopsy that progressively uncloaks this challenging cardiomyopathy. Incorporating deformation may substantially increase the gain at the cost of non-additional technical demands.
Once again, strain analysis may bridge the gap between ultrastructure and clinical events. Even though the histological basis of myocardial segments with abnormal deformation is not yet completely defined, longitudinal strain clearly seems to be on its way to be included in the SCD risk models of HCM (Figure 1).
Conflicts of interest: N.C. is on the advisory board of Mavacamten and receives consulting fees. J.A. has no conflict of interest.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal - Cardiovascular Imaging or of the European Society of Cardiology.



