-
PDF
- Split View
-
Views
-
Cite
Cite
Mihai Strachinaru, Daniel J Bowen, Alina Constatinescu, Olivier C Manintveld, Jasper J Brugts, Marcel L Geleijnse, Kadir Caliskan, Transhepatic echocardiography: a novel approach for imaging in left ventricle assist device patients with difficult acoustic windows, European Heart Journal - Cardiovascular Imaging, Volume 21, Issue 5, May 2020, Pages 491–497, https://doi.org/10.1093/ehjci/jeaa002
Close - Share Icon Share
Abstract
A significant proportion of left ventricle assist device (LVAD) patients have very difficult transthoracic echocardiographic images. The aim of this study was to find an echocardiographic window which would provide better visualization of the heart in LVAD patients with limited acoustic windows.
Based on the anatomic relationships in LVAD patients, a right intercostal transhepatic approach was proposed. By using a computer simulator, we searched for the appropriate probe orientation. Further, 15 ambulatory LVAD patients (age 56 ± 15 years, 73% males) underwent two echocardiographic studies: one normal transthoracic echocardiography following the institutional protocol (Echo 1) and a second study which included the transhepatic approach (Echo 2). The two exams were performed by two different sonographers and the results validated by a third observer for agreement. The transhepatic intercostal window was feasible in all patients, with an image quality allowing good visualization of structures in 93%. Precise quantification of the left ventricular (LV) and right ventricular (RV) function was achieved more often in the Echo 2 (10 vs. 3 patients for LV, P = 0.03 and 14 vs. 8 patients for RV, P = 0.04). A significant difference existed also in the quantification of the LVAD inflow cannula flow by pulsed Doppler (11 patients in Echo 2 vs. 3 patients in Echo 1, P = 0.009).
This is the first study describing a new echocardiographic window in LVAD patients. The transhepatic window may provide better quantification of left and RV dimensions and function and improvement in Doppler interrogation of the inflow cannula.
Introduction
Left ventricular assist devices (LVAD) are increasingly used in the treatment of end-stage heart failure.1 It can be used as a bridge to other treatment options (bridge-to-decision, heart transplantation, or even recovery) or as destination therapy.2–4 Current guidelines recommend the routine use of echocardiography in the assessment and follow-up of LVAD patients.5,6 However, a significant proportion of these patients have very difficult transthoracic echocardiographic images, because of the interposition of implanted materials and the extensive post-operative changes in the intrathoracic anatomy.7 A possible approach to improving the quality of the image is enhance visualization with the use of left ventricular (LV) contrast agents.8 Unfortunately, LV contrast agents cannot improve the visualization of the heart when the ultrasound is blocked by hard implanted material.
In this study, we describe our clinical experience with the transhepatic echocardiographic approach in consecutive LVAD patients, aiming to overcome difficult imaging due to the interposition of implanted materials (LVAD pump, inflow cannula, and outflow graft) between the probe and the heart. The hypothesis is that this new echocardiographic window may provide complementary information in cases where usual ultrasound imaging is severely limited.
Methods
As noted above, echocardiographic windows may be severely limited in LVAD patients. Therefore, in our imaging group, a lot of effort was directed towards improving the echocardiographic imaging in these patients.8 Based on a study of the anatomical relationships between the heart, its surrounding organs, and the LVAD system, derived from multiplane computed tomography images of LVAD patients, we proposed a right intercostal transhepatic approach. This view was chosen by investigating the possible echocardiographic windows that allowed interrogation of the heart while avoiding the implanted material (LVAD pump and drivelines, see Figure 1). We then used computer simulations as a theoretical guide in order to reach an optimal probe position and orientation that would allow to reproduce modified classical echocardiographic views (Figures 1and2, Supplementary data online, Movies S1 and S2). Computer simulations were performed by using the Heartworks simulation software (HeartWorks Simulator v2.0.60.0 by Intelligent Ultrasound, Cardiff, UK). In patients, the right intercostal transhepatic window was obtained by first finding the subcostal four-chamber view, and then sliding along the first adjacent intercostal space to the right, while maintaining the probe rotation and the four-chamber image (Figures 1and2, Supplementary data online, Movie S1). The aim was to obtain a four-chamber view, avoiding the hard reflectors, usually located between the heart and the thorax wall. For optimal alignment with the heart axis, it may be necessary to go one intercostal space higher, in order to obtain an ‘inverted four-chamber view’ (Supplementary data online, Movie S1). The right ventricular (RV) view was obtained by 30–60° clockwise rotation and anterior tilting of the probe (Figure 2, Supplementary data online, Movies S2 and S4).
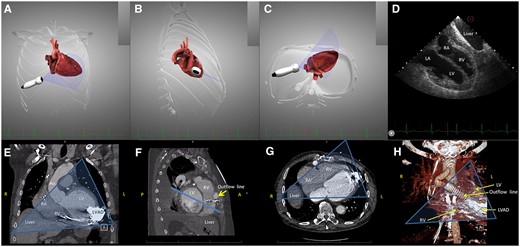
The right intercostal transhepatic imaging plane, demonstrated on the simulator (HeartWorks v2.0.60.0, Inventive Medical Ltd, MedaPhor Group plc 2019, UK) and on computed tomography on one LVAD patient. (A–C) Computer simulated direction of the probe and imaging plane in order to obtain a four-chamber view: (A) frontal plane; (B) right lateral view; (C) inferior view; (D) the computer simulated echocardiography demonstrating a modified four-chamber view, the liver is also in the imaging plane; (E) computed tomography angiogram (CTA) of an LVAD patient, the transhepatic echocardiography imaging plane is drawn over the image as a transparent triangle in the frontal (A, anterior) plane; and (F) side view of CTA images demonstrating the interposition of the outflow line between the heart and the chest wall. The echocardiography imaging plane, drawn over the image as a line, is situated posterior to the outflow LVAD line, and intersects only the heart cavities; (G) inferior view of the same patient, the transhepatic echocardiography imaging plane is drawn over the image as a transparent triangle. The LVAD is situated in the far field; and (H) 3D reconstruction from CTA images of the same LVAD patient, in the frontal (A, anterior) plane. Bone structures have been excluded, in order to allow visualization of the outflow line, situated between the heart and the chest wall. The echocardiography imaging plane, drawn over the image as a transparent triangle, is situated posterior to the outflow LVAD line. LA, left atrium; LV, left ventricle; LVAD, Left ventricle assist device; RA, right atrium; RV, right ventricle.
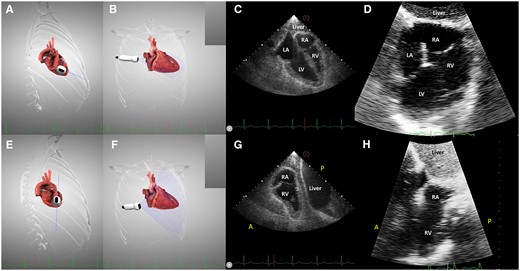
The transhepatic four-chamber view (A–D) and right ventricular view (E–H). (A and B) imaging plane and probe position demonstrated on the simulator; (C) virtual transhepatic echocardiography, four-chamber view; (D) real transhepatic echocardiography, the same incidence on a LVAD patient; (E and F) clockwise rotation of the probe and anterior tilting to obtain the right ventricular view; (G) virtual transhepatic echocardiography, right ventricular view; and (H) real transhepatic echocardiography, the right ventricular view on an LVAD patient.
This approach has been used exclusively for the LVAD patients having extremely poor or no transthoracic windows.
Study population
We prospectively included all consecutive LVAD patients, alive in 2018–19, in whom a transhepatic window was included as part of the local echocardiography protocol, because of the difficulty in appropriately imaging these patients.
Since March 2016, in our tertiary referral centre, only HeartMate 3 LVAD is implanted as bridge-to-transplant or destination therapy, before this date only HeartMate 2 were used. This pilot study was approved by the institutional Medical Ethics Committee and conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from every participant.
Inclusion criteria:
age >17 years,
stable clinical status, and
impossibility to obtain an echocardiographic apical view or very poor quality of transthoracic imaging.
Exclusion criteria:
restricted access to the classical echocardiographic windows due to incomplete healing, complications, or pain and
clinical instability.
Echocardiography
Two echocardiographic studies were performed, using a Philips EPIQ 7C ultrasound system (Philips Medical Systems, Bothell, WA, USA), equipped with an X5-1 transducer. At the first visit, each subject was imaged by one of the two sonographers in the study (M.S.—highly experienced imaging cardiologist specialized in LVAD echocardiography and D.B.—highly experienced sonographer specialized in LVAD echocardiography). At the second visit, the subjects were imaged by the second sonographer. The time interval between the two exams was variable, coupled to the outpatient clinic follow-up visits.
D.B. performed a complete echocardiographic examination (noted with Echo 1 but not necessarily the first in order), following an extensive internal protocol, based on the American Society of Echocardiography recommendations.6 In short, this protocol investigates the heart in the following echocardiographic windows:
parasternal (LV long and short axis, modified long axis for the visualization of the ascending aorta, RV long axis),
apical (LV focused, with all classical views; RV focused with multiplane and rotational imaging9),
subcostal long and short axis, and
suprasternal and right parasternal for visualization of the outflow cannula.
M.S. performed a second study protocol (noted Echo 2) based on the same views as D.B., to which was added a right intercostal transhepatic approach, focused on the LV and RV.
In both studies (Echo 1 and Echo 2) the feasibility of each window was noted, as well as the structures visualized. The quality of the image in transhepatic view was assessed using a 4-grade scale (1: good, clear visualization of all structures; 2: acceptable, structures can be visualized but there is excessive noise and/or some elements are not clearly defined; 3: bad, some structures are not visible; and 4: null). If a certain parameter could be obtained in multiple views (e.g. the RV function could be estimated in the apical or subcostal windows, or the outflow cannula could be imaged in parasternal or suprasternal position), only the view allowing most optimal quantification and alignment with the structures was retained.
The two examinations (Echo 1 and 2) were performed in a reciprocally blinded manner by the two sonographers in the study. Each study result was initially evaluated by the performing sonographer (D.B. and M.S., respectively), and validated for agreement by K.C. and M.G. (highly experienced cardiologists).
Statistics
Continuous variables were represented as mean ± standard deviation. Categorical data are presented as absolute number and percentages. For comparison of normally distributed continuous variables, we used the dependent or independent means t-test when appropriate. For comparison of proportions, the χ2 test or the Fisher’s exact test was used.
Every statistical analysis was performed using the Statistical Package for Social Sciences version 25 (IBM SPSS Statistics for Windows, Armonk, NY, USA). Testing was done two-sided and considered significant if the P-value was smaller than 0.05.
Results
During 2018–19, 43 different LVAD patients were investigated by echocardiography in the outpatient clinic and 15 were included in this pilot study. Their general characteristics are presented in Table 1. Male gender was predominant 73%, with an average age of 56 ± 15 years. Fourteen subjects had a HeartMate 3 device, and one HeartMate 2. Most of the subjects (80%) had intracavitary leads, mostly implantable cardiac-defibrillators (ICDs). Other thoracic surgical procedures (valvular, coronary bypass, and re-LVAD) were also present in this group (Table 1). They were not presented in a cumulative manner since more instances were present simultaneously in some subjects.
| Characteristics . | Data . |
|---|---|
| Age (years) | 56 ± 15 |
| Male gender | 11 (73%) |
| Height (cm) | 177 ± 10 |
| Weight (kg) | 85 ± 18 |
| BMI (kg/m2) | 27 ± 4 |
| Systolic blood pressure at inclusion (mmHg) | 106 ± 12 |
| Diastolic blood pressure at inclusion (mmHg) | 74 ± 7 |
| Ischaemic cardiomyopathy | 5 (33%) |
| CABG | 1 (7%) |
| Re-LVAD | 1 (7%) |
| Valve procedure (any position, surgical or percutaneous) | 5 (33%) |
| Other implantable devices (PM and ICD) | 12 (80%) |
| Time interval since LVAD implantation (years) | 1.7 ± 0.9 |
| Time interval between Echo 1 and Echo 2 (months) | 3.3 ± 1.6 |
| Characteristics . | Data . |
|---|---|
| Age (years) | 56 ± 15 |
| Male gender | 11 (73%) |
| Height (cm) | 177 ± 10 |
| Weight (kg) | 85 ± 18 |
| BMI (kg/m2) | 27 ± 4 |
| Systolic blood pressure at inclusion (mmHg) | 106 ± 12 |
| Diastolic blood pressure at inclusion (mmHg) | 74 ± 7 |
| Ischaemic cardiomyopathy | 5 (33%) |
| CABG | 1 (7%) |
| Re-LVAD | 1 (7%) |
| Valve procedure (any position, surgical or percutaneous) | 5 (33%) |
| Other implantable devices (PM and ICD) | 12 (80%) |
| Time interval since LVAD implantation (years) | 1.7 ± 0.9 |
| Time interval between Echo 1 and Echo 2 (months) | 3.3 ± 1.6 |
BMI, body mass index; CABG, coronary artery bypass graft; ICD, implantable cardiac-defibrillator; LVAD, left ventricle assist device; PM, pacemaker.
| Characteristics . | Data . |
|---|---|
| Age (years) | 56 ± 15 |
| Male gender | 11 (73%) |
| Height (cm) | 177 ± 10 |
| Weight (kg) | 85 ± 18 |
| BMI (kg/m2) | 27 ± 4 |
| Systolic blood pressure at inclusion (mmHg) | 106 ± 12 |
| Diastolic blood pressure at inclusion (mmHg) | 74 ± 7 |
| Ischaemic cardiomyopathy | 5 (33%) |
| CABG | 1 (7%) |
| Re-LVAD | 1 (7%) |
| Valve procedure (any position, surgical or percutaneous) | 5 (33%) |
| Other implantable devices (PM and ICD) | 12 (80%) |
| Time interval since LVAD implantation (years) | 1.7 ± 0.9 |
| Time interval between Echo 1 and Echo 2 (months) | 3.3 ± 1.6 |
| Characteristics . | Data . |
|---|---|
| Age (years) | 56 ± 15 |
| Male gender | 11 (73%) |
| Height (cm) | 177 ± 10 |
| Weight (kg) | 85 ± 18 |
| BMI (kg/m2) | 27 ± 4 |
| Systolic blood pressure at inclusion (mmHg) | 106 ± 12 |
| Diastolic blood pressure at inclusion (mmHg) | 74 ± 7 |
| Ischaemic cardiomyopathy | 5 (33%) |
| CABG | 1 (7%) |
| Re-LVAD | 1 (7%) |
| Valve procedure (any position, surgical or percutaneous) | 5 (33%) |
| Other implantable devices (PM and ICD) | 12 (80%) |
| Time interval since LVAD implantation (years) | 1.7 ± 0.9 |
| Time interval between Echo 1 and Echo 2 (months) | 3.3 ± 1.6 |
BMI, body mass index; CABG, coronary artery bypass graft; ICD, implantable cardiac-defibrillator; LVAD, left ventricle assist device; PM, pacemaker.
There was no significant difference in the feasibility per echocardiographic window between the two exams (Table 2), except of course for the transhepatic window, which was specific to Echo 2. The transhepatic window was obtained in all study subjects (Table 2), with an image quality of 1(good) in eight (53%) patients, two (acceptable) in six (40%) subjects, and three (bad) in one patient (7%). The imaging depth was 26 ± 4 cm.
| Echocardiographic window . | Echo 1 . | Echo 2 . | P . |
|---|---|---|---|
| Parasternal | 14 (93%) | 14 (93%) | — |
| Apical LV | 4 (27%) | 4 (27%) | — |
| Apical RV | 8 (53%) | 9 (60%) | 1 |
| Subcostal | 11 (73%) | 11 (73%) | — |
| Suprasternal/right parasternal | 12 (80%) | 14 (93%) | 0.6 |
| Transhepatic | 0 | 15 | — |
| Transhepatic imaging depth (cm) | — | 26 ± 4 | — |
| Transhepatic imaging frame rate (Hz) | — | 48 ± 10 | — |
| Echocardiographic window . | Echo 1 . | Echo 2 . | P . |
|---|---|---|---|
| Parasternal | 14 (93%) | 14 (93%) | — |
| Apical LV | 4 (27%) | 4 (27%) | — |
| Apical RV | 8 (53%) | 9 (60%) | 1 |
| Subcostal | 11 (73%) | 11 (73%) | — |
| Suprasternal/right parasternal | 12 (80%) | 14 (93%) | 0.6 |
| Transhepatic | 0 | 15 | — |
| Transhepatic imaging depth (cm) | — | 26 ± 4 | — |
| Transhepatic imaging frame rate (Hz) | — | 48 ± 10 | — |
| Echocardiographic window . | Echo 1 . | Echo 2 . | P . |
|---|---|---|---|
| Parasternal | 14 (93%) | 14 (93%) | — |
| Apical LV | 4 (27%) | 4 (27%) | — |
| Apical RV | 8 (53%) | 9 (60%) | 1 |
| Subcostal | 11 (73%) | 11 (73%) | — |
| Suprasternal/right parasternal | 12 (80%) | 14 (93%) | 0.6 |
| Transhepatic | 0 | 15 | — |
| Transhepatic imaging depth (cm) | — | 26 ± 4 | — |
| Transhepatic imaging frame rate (Hz) | — | 48 ± 10 | — |
| Echocardiographic window . | Echo 1 . | Echo 2 . | P . |
|---|---|---|---|
| Parasternal | 14 (93%) | 14 (93%) | — |
| Apical LV | 4 (27%) | 4 (27%) | — |
| Apical RV | 8 (53%) | 9 (60%) | 1 |
| Subcostal | 11 (73%) | 11 (73%) | — |
| Suprasternal/right parasternal | 12 (80%) | 14 (93%) | 0.6 |
| Transhepatic | 0 | 15 | — |
| Transhepatic imaging depth (cm) | — | 26 ± 4 | — |
| Transhepatic imaging frame rate (Hz) | — | 48 ± 10 | — |
Both exams allowed qualitative evaluation of the left and RV function in similar percentages (Table 3). The outflow cannula flow was also similarly imaged. Quantification of LV (ejection fraction by Simpson method) and RV function (tricuspid annular plane systolic excursion) was achieved more often in the Echo 2 (10 vs. 3 patients for LV, P = 0.03 and 14 vs. 8 patients for RV, P = 0.04). Figure 3 demonstrates the RV function assessment in transhepatic view. A significant difference existed also in the quantification of the LVAD inflow cannula (Figure 4) flow by pulsed Doppler (11 patients in Echo 2 vs. 3 patients in Echo 1, P = 0.009).
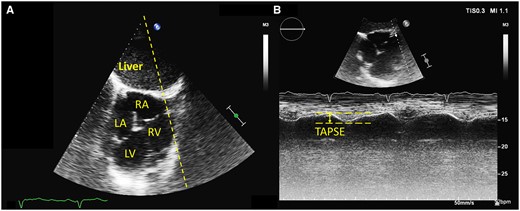
Right ventricular function assessed in transhepatic view by tricuspid annular plane systolic excursion (TAPSE). (A) general transhepatic view of an ‘inverted’ four-chamber view. The TAPSE M-mode line is drawn over the image (dotted line). (B) TAPSE measurement from the transhepatic view. The systolic excursion is also inverted (downward movement in systole).

Colour Doppler and pulsed-wave Doppler interrogation of the LVAD inflow cannula. (A) The inflow cannula is situated in the far field, in the LV apex. (B) By using pulsed-wave Doppler, we can measure the inflow velocities.
| Parameter . | Feasibility Echo 1 . | Feasibility Echo 2 . | P . | View where the parameter was acquired Echo 1 . | View where the parameter was acquired Echo 2 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Para sternal . | Apical . | Sub costal . | Supra sternal . | Para sternal . | Apical . | Sub costal . | Supra sternal . | Transhepatic . | ||||
| LV function qualitative | 14 (93%) | 15 (100%) | 1 | 10 (67%) | 4 (27%) | 0 | 0 | 1 (7%) | 4 (27%) | 0 | 0 | 10 (67%) |
| LVEF Simpson (monoplan) | 3 (20%) | 10 (67%) | 0.03 | 0 | 3 (20%) | 0 | 0 | 0 | 4 (27%) | 0 | 0 | 6 (40%) |
| RV function qualitative | 12 (80%) | 15 (100%) | 0.2 | 1 (7%) | 8 (53%) | 3 (20%) | 0 | 0 | 9 (60%) | 0 | 0 | 6 (40%) |
| TAPSE | 8 (53%) | 14 (93%) | 0.04 | 0 | 8 (53%) | 0 | 0 | 0 | 9 (60%) | 0 | 0 | 5 (33%) |
| Inflow cannula flow | 3 (20%) | 11 (73%) | 0.009 | 0 | 2 (13%) | 1 (7%) | 0 | 1 (7%) | 1 (7%) | 0 | 0 | 9 (60%) |
| Outflow cannula flow | 12 (80%) | 15 (100%) | 0.2 | 2 (13%) | 0 | 0 | 10 (67%) | 1 (7%) | 0 | 0 | 14 (93%) | 0 |
| Parameter . | Feasibility Echo 1 . | Feasibility Echo 2 . | P . | View where the parameter was acquired Echo 1 . | View where the parameter was acquired Echo 2 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Para sternal . | Apical . | Sub costal . | Supra sternal . | Para sternal . | Apical . | Sub costal . | Supra sternal . | Transhepatic . | ||||
| LV function qualitative | 14 (93%) | 15 (100%) | 1 | 10 (67%) | 4 (27%) | 0 | 0 | 1 (7%) | 4 (27%) | 0 | 0 | 10 (67%) |
| LVEF Simpson (monoplan) | 3 (20%) | 10 (67%) | 0.03 | 0 | 3 (20%) | 0 | 0 | 0 | 4 (27%) | 0 | 0 | 6 (40%) |
| RV function qualitative | 12 (80%) | 15 (100%) | 0.2 | 1 (7%) | 8 (53%) | 3 (20%) | 0 | 0 | 9 (60%) | 0 | 0 | 6 (40%) |
| TAPSE | 8 (53%) | 14 (93%) | 0.04 | 0 | 8 (53%) | 0 | 0 | 0 | 9 (60%) | 0 | 0 | 5 (33%) |
| Inflow cannula flow | 3 (20%) | 11 (73%) | 0.009 | 0 | 2 (13%) | 1 (7%) | 0 | 1 (7%) | 1 (7%) | 0 | 0 | 9 (60%) |
| Outflow cannula flow | 12 (80%) | 15 (100%) | 0.2 | 2 (13%) | 0 | 0 | 10 (67%) | 1 (7%) | 0 | 0 | 14 (93%) | 0 |
Significant P-values (<0.05) were highlighted in bold. LV, left ventricle; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane excursion.
| Parameter . | Feasibility Echo 1 . | Feasibility Echo 2 . | P . | View where the parameter was acquired Echo 1 . | View where the parameter was acquired Echo 2 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Para sternal . | Apical . | Sub costal . | Supra sternal . | Para sternal . | Apical . | Sub costal . | Supra sternal . | Transhepatic . | ||||
| LV function qualitative | 14 (93%) | 15 (100%) | 1 | 10 (67%) | 4 (27%) | 0 | 0 | 1 (7%) | 4 (27%) | 0 | 0 | 10 (67%) |
| LVEF Simpson (monoplan) | 3 (20%) | 10 (67%) | 0.03 | 0 | 3 (20%) | 0 | 0 | 0 | 4 (27%) | 0 | 0 | 6 (40%) |
| RV function qualitative | 12 (80%) | 15 (100%) | 0.2 | 1 (7%) | 8 (53%) | 3 (20%) | 0 | 0 | 9 (60%) | 0 | 0 | 6 (40%) |
| TAPSE | 8 (53%) | 14 (93%) | 0.04 | 0 | 8 (53%) | 0 | 0 | 0 | 9 (60%) | 0 | 0 | 5 (33%) |
| Inflow cannula flow | 3 (20%) | 11 (73%) | 0.009 | 0 | 2 (13%) | 1 (7%) | 0 | 1 (7%) | 1 (7%) | 0 | 0 | 9 (60%) |
| Outflow cannula flow | 12 (80%) | 15 (100%) | 0.2 | 2 (13%) | 0 | 0 | 10 (67%) | 1 (7%) | 0 | 0 | 14 (93%) | 0 |
| Parameter . | Feasibility Echo 1 . | Feasibility Echo 2 . | P . | View where the parameter was acquired Echo 1 . | View where the parameter was acquired Echo 2 . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Para sternal . | Apical . | Sub costal . | Supra sternal . | Para sternal . | Apical . | Sub costal . | Supra sternal . | Transhepatic . | ||||
| LV function qualitative | 14 (93%) | 15 (100%) | 1 | 10 (67%) | 4 (27%) | 0 | 0 | 1 (7%) | 4 (27%) | 0 | 0 | 10 (67%) |
| LVEF Simpson (monoplan) | 3 (20%) | 10 (67%) | 0.03 | 0 | 3 (20%) | 0 | 0 | 0 | 4 (27%) | 0 | 0 | 6 (40%) |
| RV function qualitative | 12 (80%) | 15 (100%) | 0.2 | 1 (7%) | 8 (53%) | 3 (20%) | 0 | 0 | 9 (60%) | 0 | 0 | 6 (40%) |
| TAPSE | 8 (53%) | 14 (93%) | 0.04 | 0 | 8 (53%) | 0 | 0 | 0 | 9 (60%) | 0 | 0 | 5 (33%) |
| Inflow cannula flow | 3 (20%) | 11 (73%) | 0.009 | 0 | 2 (13%) | 1 (7%) | 0 | 1 (7%) | 1 (7%) | 0 | 0 | 9 (60%) |
| Outflow cannula flow | 12 (80%) | 15 (100%) | 0.2 | 2 (13%) | 0 | 0 | 10 (67%) | 1 (7%) | 0 | 0 | 14 (93%) | 0 |
Significant P-values (<0.05) were highlighted in bold. LV, left ventricle; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane excursion.
Discussion
In this study, we found that in LVAD patients with very poor traditional echocardiographic windows: (i) the novel transhepatic intercostal approach for echocardiographic imaging is feasible; (ii) resulting in a higher rate of LVAD patients to have a precise quantification of LV and RV function; and (iii) a higher proportion of patients to benefit from Doppler evaluation of LVAD inflow cannula.
LVADs are increasingly being used in the treatment of advanced heart failure, as destination therapy, bridge-to-decision, or as bridge to transplantation.1 Recovery is also a possible scenario in very limited, selected cases when the LVAD may even be explanted.10 Essential to these decisions, as well as for the follow-up of these patients, is being able to reliably assess the function of the heart chamber and the function of the LVAD pump. Currently, transthoracic echocardiography is the main imaging method used in the follow-up of LVAD patients,5,6 due to its low cost, portability, repeatability, and lack of adverse events. However, in most of the LVAD patients, echocardiographic imaging is severely limited by the implanted material (LVAD, drivelines, and connections), and this limitation is possibly under-reported.7,8
Given the strengths and limitations mentioned above, we aimed to find an echocardiographic window, which would avoid the interposition of implanted material between the heart and the probe. Because of the anatomic relationships between the heart, lungs, the LVAD device, and its connections, a transhepatic approach is practically the only imaging plane corresponding to these conditions (see Figure 1E–H). By using a computer simulator study, we were able to search for a probe position, rotation, and angulation needed in order to image the heart cavities from that position. This was followed by in vivo testing, where the window proved to be highly feasible (100% in our group) with acceptable or good image quality (according to our definition, which takes into account the imaging depth and the transhepatic passage) in 93%. However, the only LV view investigated in the transhepatic window was the four-chamber view. This is because the manual probe rotation for a ventricle situated in the far field and slightly angulated did not result in obtaining full-length two-chamber or three-chamber view. The same problems were encountered when using simultaneous multiplane or digital rotation of the imaging plane, where the quality of the rotated image is also known to be decreased.11
The transhepatic window allows for longitudinal views of the LV and RV, but the heart is situated very deep, leading to a significant drop in resolution and frame rates achievable (Figures 2and3, Table 2). The quality of the image obtained was considered acceptable in this particular group of patients having very limited access in the classical windows (Table 2), because it was sufficient to allow visualization of the cardiac structures and the assessment of function and flow (Figures 3and4), with higher success rate than by classical approach (Table 3). The ultrasound, as shown in Figures 1and2, must first traverse the hepatic parenchyma, which leads to loss of energy through attenuation.12 In our study group, with an average body mass index of 27 ± 4 and a long-standing history of heart failure, the incidence of a fatty liver was of course lower, but variable degrees of liver fibrosis remain possible.13 On the contrary, liver oedema may favour the penetration of ultrasound. Transhepatic imaging has already been proposed as a good alternative for vena cava inferior assessment during heart surgery.14
The possible presence of large implanted material in the right heart (valve replacement, tricuspid valvuloplasty, and right heart mechanical circulatory support) may significantly reduce the feasibility of this method. Defibrillator leads, although very frequent in our study group, did not seem to modify the results. In this study, we demonstrate that the transhepatic imaging plane has the potential to offer an acceptable visualization of the heart in cases where the standard transthoracic windows are unusable. This is proven by the significantly higher number of patients where precise quantification of LV and RV function was possible by using a transhepatic window. This may prove crucial in cases where therapeutic decisions rely on precise determination of the heart dimensions or function (i.e. new onset of symptoms, unexplained alarms, possible LV recovery). It may also reduce the need for more expensive, more invasive, or higher risk investigations4–7 (computed tomography, angiography, and even transoesophageal echocardiography). In this study, none of the subjects benefited from the adjunction of LV contrast, which may enhance visualization of the heart chamber.15 Our research group has demonstrated an added value of contrast imaging in LVAD patients.8 However, high imaging depth and the interposition of contrast-filled structures (here the liver, atria, and/or the RV) may reduce the benefit of contrast for LV visualization. This may be subject for future research.
Furthermore, the very elusive inflow cannula7 could also be interrogated in 73% of subjects as opposed to 20% by traditional echocardiography (P = 0.009). These numbers are relatively low in comparison with old data reported on HeartMate 2 devices, which are more than 80% in the global LVAD population.16,17 There are no available reports on the real-life feasibility of the transthoracic Doppler interrogation of the inflow cannula in HeartMate 3 patients. Experts agree, however, that in new-generation LVADs the inflow cannula may be more difficult to image due to specific Doppler artefacts.6,18 On the contrary, the observed feasibility for the outflow cannula was excellent and similar for both echocardiographic studies (Table 3). Also, our study population consisted of particularly difficult patients, as reflected by the very low feasibility of the apical window (27%, see Table 2). Being able to interrogate, both the inflow and outflow cannulas with regular transthoracic echocardiography is a notable advantage in these patients. Most of the adaptive changes in the LVAD function, as well as a significant proportion of the alarms, are related to changes in flow and can be understood by using a combination of imaging and LVAD flow readings.1,5,6
Limitations and future perspective
This is a small pilot study describing a novel approach to echocardiographic imaging in challenging LVAD patients, with retrospective data analysis, enrolling only a limited number of patients. This new window may provide essential information for patient management when the classical echocardiographic views are non-diagnostic. Studies in larger population groups may further demonstrate the added value of the transhepatic echocardiographic approach and the practical benefit for LVAD patients on long-term follow-up.
In this monocentric study, the echocardiograms were performed by two highly skilled imaging experts, specialized in LVAD imaging. It is possible that the real-life feasibility of both the traditional ultrasound protocol and the new transhepatic window be lower, especially in particularly challenging cases. The transhepatic window is now systematically used in all our LVAD patients for their follow-up examinations. Based on this experience, we noticed that the learning curve for this new window was around 20 first-hand echocardiography examinations for a sonographer already familiar with the pathology. Obtaining the transhepatic images requires around five additional minutes to the standard LVAD examination protocol.
Most of our patients had HeartMate 3 devices (only one HeartMate 2). It is not sure whether the current results may be extrapolated to other type of LVAD systems. However all mainstream long-term LVAD types (HeartMate 2, HeartMate 3, and HeartWare) closely resemble the HeartMate 3 with respect to their implantation in the thorax; therefore, we expect similar benefits of the transhepatic approach. The method should be tested in future studies in patients implanted with all long-term LVAD models.
Conclusion
This is the first study describing a novel echocardiographic window for appropriate cardiac imaging of LVAD patients with limited acoustic access. The transhepatic window provided better quantification of LV and RV function and higher success rate for Doppler interrogation of the inflow cannula in patients with LVAD and difficult transthoracic echocardiographic image.
Conflict of interest: none declared.



