-
PDF
- Split View
-
Views
-
Cite
Cite
Maurizio Galderisi, Erwan Donal, Julien Magne, Francesco Lo Iudice, Eustachio Agricola, Leyla Elif Sade, Matteo Cameli, Ehud Schwammenthal, Nuno Cardim, Bernard Cosyns, Andreas Hagendorff, Alexandar N Neskovic, Josè Luis Zamorano, Patrizio Lancellotti, Gilbert Habib, Thor Edvardsen, Bogdan A Popescu, Rationale and design of the EACVI AFib Echo Europe Registry for assessing relationships of echocardiographic parameters with clinical thrombo-embolic and bleeding risk profile in non-valvular atrial fibrillation, European Heart Journal - Cardiovascular Imaging, Volume 19, Issue 3, March 2018, Pages 245–252, https://doi.org/10.1093/ehjci/jex322
Close - Share Icon Share
Abstract
The European Society of Cardiology (ESC) guidelines for management of atrial fibrillation (AF) recommend the use of CHA2DS2VASc risk score for assessment of thromboembolic (TE) risk, whereas the stratification of bleeding risk should be obtained by HAS-Bleed to balance the most appropriate anticoagulation (OAC) therapy. However, men with CHA2DS2VASc score = 1 and women with CHA2DS2VASc = 2, who are at intermediate TE risk, represent a grey zone where guidelines do not provide a definite OAC indication. Accordingly, implementation of risk stratification with echocardiography could be extremely useful. Both prospective and cross-sectional studies on transthoracic echocardiography (TTE) prediction of TE events and studies utilizing transoesophageal echocardiographic parameters as surrogate markers of TE events makes sustainable the hypothesis that echocardiography could improve TE prediction in non-valvular AF. Moreover, considering the close association of AF and stroke, all echo-Doppler parameters that have shown to predict AF onset and recurrence could be useful also to predict TE events in this clinical setting. Accordingly, EACVI AFib Echo Europe Registry has been designed as an observational, cross-sectional study, with the aim of evaluating: (i) left atrial (LA) size and function together with left ventricular geometry, systolic and diastolic functions in paroxysmal, persistent, and permanent AF; (ii) relationships of structural/functional parameters with clinical TE and bleeding risk profile. By the AFib Echo Europe Registry, we expect to collect data on echocardiographic phenotype of patients with AF. The large data set accumulated will be useful to test the level of agreement of different echocardiographic measurements with the available risk scores.
Introduction
Atrial fibrillation (AF) is associated with a five-fold increased risk of developing ischaemic stroke, and 20–30% of all ischaemic strokes are directly related to AF.1,2 Oral anticoagulation (OAC) is able to reduce mortality due to ischaemic stroke in AF,3 but the protective effect against thromboembolic (TE) events needs to be balanced with the bleeding risk induced by these drugs. The most recent guidelines recommend the use of CHA2DS2VASc risk score for assessment of TE risk (both cerebral and peripheral) and establishing the OAC need in non-valvular AF. Stratification of bleeding risk should also be obtained, with the aim of identifying high-risk patients and eliminate modifiable risk factors.4 However, a grey zone exists for men with CHA2DS2VASc score = 1 and women with CHA2DS2VASc = 2, who are at intermediate TE risk. Guidelines do not provide a strong indication for OAC in these two categories, suggesting that the choice should be individualized and based on patient’s preference. Nevertheless, TE risk is not negligible in this grey zone, with an event rate per year estimated to be up to 2.8% according to case series.5–7 This setting of patients represents an even more delicate population if they also present a high bleeding risk. In the absence of strong guideline indications, the use of OAC can expose these patients to haemorrhagic complications while its denial induces avoidable TE events.
The implementation of clinical risk stratification with other tools could be therefore extremely useful. Despite considered as an extremely valuable technique for AF in a recent EACVI Expert Consensus document,8 no definite indication exists in the current guidelines about the use of echocardiographic parameters for estimating TE risk in AF patients.
Studies on transthoracic echocardiography prediction of TE events
Prospective studies. The most relevant studies testing the predictive value of echocardiography in terms of incidence of TE events, independent on clinical risk profile, are summarized in Table 1. Several factors such as differences in inclusion criteria, choice of parameters to be tested, and clinical risk factors considered as covariates could have contributed to the heterogeneity of results obtained. Left atrial (LA) dilatation emerged as an independent predictor of TE events in non-valvular AF patients not undergoing OAC/antiplatelets drugs9 and after surgical maze procedure for sinus rhythm restoration.10 LA dilatation was an independent predictor of stroke recurrence in AF patients admitted to the hospital for acute ischaemic stroke.11 Left ventricular (LV) systolic dysfunction was also a predictor of thromboembolism in non-valvular AF case series.9,12 However, two prospective studies were not able to demonstrate an independent predictive value against TE events in any of the analysed echo parameters in non-valvular AF.13,14 Worthy of note, none of these prospective studies focused patients at intermediate TE risk derived by clinical risk scores.
Table 1Prospective studies assessing the predictive value of echocardiographic parameters vs. ischaemic stroke/systemic thromboembolism
. No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Median follow-up . No. of events . Results . Gupta et al.14 971 Non-valvular AF, CHADS2 ≥2, anticoagulation with edoxaban or warfarin Thromboembolic events, death for any cause LV EF, LVMi, LV EDVi, LAD, LAVi, LA EF, E/e′ 2.5 years 48 No echo parameter significantly predicted the endpoint Buber et al.10 150 AF patients undergoing maze surgical procedure. Anticoagulation suspended 3 months after procedure Thromboembolic stroke LAVi, absence of A wave at transmitral PW Doppler 17 months if A wave present, 19 months if A velocity absent 15 Absence of A wave predicts the endpoint in the entire population (HR 4.76; 95% CI 1.19–19.0, P = 0.02) and in patients not on anticoagulant (HR 4.18; 95% CI 1.07–16.3, P = 0.04), LAVi predicts the endpoint in the entire population (HR = 3.23; 95% CI 1.10–9.48, P = 0.03) but not in patients not on anticoagulation Ezekowitz et al.12 1066 Non-valvular AF patients Ischaemic stroke LAD, LV septal and posterior wall thickness, LVMi, LV dysfunction, MR, MV prolapse 1.6 years 78 Only LV moderate–severe systolic dysfunction is significantly associated with the endpoint at multivariate analysis (RR 2.5; 95% CI 1.5–4.4, P < 0.010) Olshansky et al.13 2474 Non-valvular AF patients after restoration of sinus rhythm Ischaemic stroke LAD, reduced LV EF, MR at least moderate >5 years 86 No echo parameters significantly associated with the endpoint at multivariate analysis Paciaroni et al.11 854 AF patients admitted for acute ischaemic stroke Recurrence of stroke/TIA, systemic embolism LA enlargement (LAD or LAVi)a 90 days 63 Severe atrial enlargement predicts the endpoint at multivariate analysis (OR 2.05; 95% CI 1.08–2.87, P = 0.027) Asinger et al.9 568 Non-valvular AF patients not on anticoagulant or antiplatelet Ischaemic stroke/systemic embolism LAD index, LVMi, LV EDD and ESD, FS, LV systolic dysfunction, severe MR 1.3 years 46 LV dysfunction (RR = 2.0; 95% CI 1.0–4.0, P < 0.05) and LAD (RR = 1.6; 95% CI 1.0–2.5, P = 0.04) are independent predictors of the endpoint . No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Median follow-up . No. of events . Results . Gupta et al.14 971 Non-valvular AF, CHADS2 ≥2, anticoagulation with edoxaban or warfarin Thromboembolic events, death for any cause LV EF, LVMi, LV EDVi, LAD, LAVi, LA EF, E/e′ 2.5 years 48 No echo parameter significantly predicted the endpoint Buber et al.10 150 AF patients undergoing maze surgical procedure. Anticoagulation suspended 3 months after procedure Thromboembolic stroke LAVi, absence of A wave at transmitral PW Doppler 17 months if A wave present, 19 months if A velocity absent 15 Absence of A wave predicts the endpoint in the entire population (HR 4.76; 95% CI 1.19–19.0, P = 0.02) and in patients not on anticoagulant (HR 4.18; 95% CI 1.07–16.3, P = 0.04), LAVi predicts the endpoint in the entire population (HR = 3.23; 95% CI 1.10–9.48, P = 0.03) but not in patients not on anticoagulation Ezekowitz et al.12 1066 Non-valvular AF patients Ischaemic stroke LAD, LV septal and posterior wall thickness, LVMi, LV dysfunction, MR, MV prolapse 1.6 years 78 Only LV moderate–severe systolic dysfunction is significantly associated with the endpoint at multivariate analysis (RR 2.5; 95% CI 1.5–4.4, P < 0.010) Olshansky et al.13 2474 Non-valvular AF patients after restoration of sinus rhythm Ischaemic stroke LAD, reduced LV EF, MR at least moderate >5 years 86 No echo parameters significantly associated with the endpoint at multivariate analysis Paciaroni et al.11 854 AF patients admitted for acute ischaemic stroke Recurrence of stroke/TIA, systemic embolism LA enlargement (LAD or LAVi)a 90 days 63 Severe atrial enlargement predicts the endpoint at multivariate analysis (OR 2.05; 95% CI 1.08–2.87, P = 0.027) Asinger et al.9 568 Non-valvular AF patients not on anticoagulant or antiplatelet Ischaemic stroke/systemic embolism LAD index, LVMi, LV EDD and ESD, FS, LV systolic dysfunction, severe MR 1.3 years 46 LV dysfunction (RR = 2.0; 95% CI 1.0–4.0, P < 0.05) and LAD (RR = 1.6; 95% CI 1.0–2.5, P = 0.04) are independent predictors of the endpoint AF, atrial fibrillation; EDD, end-diastolic diameter; EDVi, end-diastolic volume index; EF, ejection fraction; ESD, end-systolic diameter; FS, fractional shortening; LA, left atrium; LAD, left atrial anteroposterior diameter; LAVi, left atrial volume index; LV , left ventricular; LVMi, LV mass index; MR, mitral regurgitation; MV, mitral valve.
aFor LAD: mild dilatation = 39–42 mm in female/41–46 mm in male; moderate dilatation = 43–46 mm in female/47–52 mm in male; severe dilatation >46 in female/>52 in male. For LAVi: mild dilatation = 29–33 mL/m2; moderate dilatation = 34–39 mL/m2; severe dilatation <39 mL/m2.
Table 1Prospective studies assessing the predictive value of echocardiographic parameters vs. ischaemic stroke/systemic thromboembolism
. No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Median follow-up . No. of events . Results . Gupta et al.14 971 Non-valvular AF, CHADS2 ≥2, anticoagulation with edoxaban or warfarin Thromboembolic events, death for any cause LV EF, LVMi, LV EDVi, LAD, LAVi, LA EF, E/e′ 2.5 years 48 No echo parameter significantly predicted the endpoint Buber et al.10 150 AF patients undergoing maze surgical procedure. Anticoagulation suspended 3 months after procedure Thromboembolic stroke LAVi, absence of A wave at transmitral PW Doppler 17 months if A wave present, 19 months if A velocity absent 15 Absence of A wave predicts the endpoint in the entire population (HR 4.76; 95% CI 1.19–19.0, P = 0.02) and in patients not on anticoagulant (HR 4.18; 95% CI 1.07–16.3, P = 0.04), LAVi predicts the endpoint in the entire population (HR = 3.23; 95% CI 1.10–9.48, P = 0.03) but not in patients not on anticoagulation Ezekowitz et al.12 1066 Non-valvular AF patients Ischaemic stroke LAD, LV septal and posterior wall thickness, LVMi, LV dysfunction, MR, MV prolapse 1.6 years 78 Only LV moderate–severe systolic dysfunction is significantly associated with the endpoint at multivariate analysis (RR 2.5; 95% CI 1.5–4.4, P < 0.010) Olshansky et al.13 2474 Non-valvular AF patients after restoration of sinus rhythm Ischaemic stroke LAD, reduced LV EF, MR at least moderate >5 years 86 No echo parameters significantly associated with the endpoint at multivariate analysis Paciaroni et al.11 854 AF patients admitted for acute ischaemic stroke Recurrence of stroke/TIA, systemic embolism LA enlargement (LAD or LAVi)a 90 days 63 Severe atrial enlargement predicts the endpoint at multivariate analysis (OR 2.05; 95% CI 1.08–2.87, P = 0.027) Asinger et al.9 568 Non-valvular AF patients not on anticoagulant or antiplatelet Ischaemic stroke/systemic embolism LAD index, LVMi, LV EDD and ESD, FS, LV systolic dysfunction, severe MR 1.3 years 46 LV dysfunction (RR = 2.0; 95% CI 1.0–4.0, P < 0.05) and LAD (RR = 1.6; 95% CI 1.0–2.5, P = 0.04) are independent predictors of the endpoint . No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Median follow-up . No. of events . Results . Gupta et al.14 971 Non-valvular AF, CHADS2 ≥2, anticoagulation with edoxaban or warfarin Thromboembolic events, death for any cause LV EF, LVMi, LV EDVi, LAD, LAVi, LA EF, E/e′ 2.5 years 48 No echo parameter significantly predicted the endpoint Buber et al.10 150 AF patients undergoing maze surgical procedure. Anticoagulation suspended 3 months after procedure Thromboembolic stroke LAVi, absence of A wave at transmitral PW Doppler 17 months if A wave present, 19 months if A velocity absent 15 Absence of A wave predicts the endpoint in the entire population (HR 4.76; 95% CI 1.19–19.0, P = 0.02) and in patients not on anticoagulant (HR 4.18; 95% CI 1.07–16.3, P = 0.04), LAVi predicts the endpoint in the entire population (HR = 3.23; 95% CI 1.10–9.48, P = 0.03) but not in patients not on anticoagulation Ezekowitz et al.12 1066 Non-valvular AF patients Ischaemic stroke LAD, LV septal and posterior wall thickness, LVMi, LV dysfunction, MR, MV prolapse 1.6 years 78 Only LV moderate–severe systolic dysfunction is significantly associated with the endpoint at multivariate analysis (RR 2.5; 95% CI 1.5–4.4, P < 0.010) Olshansky et al.13 2474 Non-valvular AF patients after restoration of sinus rhythm Ischaemic stroke LAD, reduced LV EF, MR at least moderate >5 years 86 No echo parameters significantly associated with the endpoint at multivariate analysis Paciaroni et al.11 854 AF patients admitted for acute ischaemic stroke Recurrence of stroke/TIA, systemic embolism LA enlargement (LAD or LAVi)a 90 days 63 Severe atrial enlargement predicts the endpoint at multivariate analysis (OR 2.05; 95% CI 1.08–2.87, P = 0.027) Asinger et al.9 568 Non-valvular AF patients not on anticoagulant or antiplatelet Ischaemic stroke/systemic embolism LAD index, LVMi, LV EDD and ESD, FS, LV systolic dysfunction, severe MR 1.3 years 46 LV dysfunction (RR = 2.0; 95% CI 1.0–4.0, P < 0.05) and LAD (RR = 1.6; 95% CI 1.0–2.5, P = 0.04) are independent predictors of the endpoint AF, atrial fibrillation; EDD, end-diastolic diameter; EDVi, end-diastolic volume index; EF, ejection fraction; ESD, end-systolic diameter; FS, fractional shortening; LA, left atrium; LAD, left atrial anteroposterior diameter; LAVi, left atrial volume index; LV , left ventricular; LVMi, LV mass index; MR, mitral regurgitation; MV, mitral valve.
aFor LAD: mild dilatation = 39–42 mm in female/41–46 mm in male; moderate dilatation = 43–46 mm in female/47–52 mm in male; severe dilatation >46 in female/>52 in male. For LAVi: mild dilatation = 29–33 mL/m2; moderate dilatation = 34–39 mL/m2; severe dilatation <39 mL/m2.
Cross-sectional studies. A series of cross-sectional studies sought to identify which echocardiographic parameter could be associated with history of TE events in AF patients (Table 2). E/e′ ratio of mitral annulus had an independent association with history of stroke both in paroxysmal15 and permanent/persistent non-valvular AF.16 LA peak systolic longitudinal strain was independently associated with history of stroke in two series of non-valvular AF patients.17,18
Table 2Cross-sectional studies assessing the association of echocardiographic parameters with prevalence of ischaemic stroke/systemic thromboembolism
. No. of patients . Patients enrolled . Event assessed . Echo parameters tested . No. of events . Results . Kim et al.15 1098 Paroxysmal non valvular AF patients undergoing catheter ablation Stroke/TIA LAD, LVMi, LV EF, transmitral E velocity, e′ velocity, s′ velocity, E/e′ 114 LAD, e′ velocity, s′ velocity and E/e′ associated with stroke/TIA at univariate analyses Only E/e′ associated with stroke/TIA at multivariate analysis after controlling for clinical risk factors Lee et al.16 330 Permanent/persistent AF, LV EF > 40%, absence of severe valve disease, congenital heart disease or hypertrophic cardiomyopathy Stroke LAVi, E velocity, e′ velocity, E/e′ 50 E velocity, e′ velocity and E/e′ associated with stroke at univariate analysis. At multivariate regression analysis only E/e′ (odds ratio = 1.21, P = 0.002) associated with stroke Obokata et al.17 285 Non valvular AF patients admitted for acute stroke/embolism (N = 82) and AF control patients without stroke (n = 203) Stroke/ embolism LAVi (max), LAVi (min), LA emptying fraction, LA strain 82 LA strain independently associated with stroke/embolism at multivariate analysis Sakabe et al.19 76 Paroxysmal non-valvular AF with no risk factors or only mild hypertension Stroke E/A ratio, atrial reverse velocity, LAV 26 E/A ratio (≥1.00), AR velocity (≤25 cm/s), and LA volume (≥107 mL) are significantly associated with stroke Shih et al.18 79 Permanent non valvular AF patients Stroke LA peak strain and LA strain rate during reservoir and conduct, e′ velocity, E/e′. 20 LA peak strain and strain rate during reservoir are independently associated with stroke at multivariate analysis . No. of patients . Patients enrolled . Event assessed . Echo parameters tested . No. of events . Results . Kim et al.15 1098 Paroxysmal non valvular AF patients undergoing catheter ablation Stroke/TIA LAD, LVMi, LV EF, transmitral E velocity, e′ velocity, s′ velocity, E/e′ 114 LAD, e′ velocity, s′ velocity and E/e′ associated with stroke/TIA at univariate analyses Only E/e′ associated with stroke/TIA at multivariate analysis after controlling for clinical risk factors Lee et al.16 330 Permanent/persistent AF, LV EF > 40%, absence of severe valve disease, congenital heart disease or hypertrophic cardiomyopathy Stroke LAVi, E velocity, e′ velocity, E/e′ 50 E velocity, e′ velocity and E/e′ associated with stroke at univariate analysis. At multivariate regression analysis only E/e′ (odds ratio = 1.21, P = 0.002) associated with stroke Obokata et al.17 285 Non valvular AF patients admitted for acute stroke/embolism (N = 82) and AF control patients without stroke (n = 203) Stroke/ embolism LAVi (max), LAVi (min), LA emptying fraction, LA strain 82 LA strain independently associated with stroke/embolism at multivariate analysis Sakabe et al.19 76 Paroxysmal non-valvular AF with no risk factors or only mild hypertension Stroke E/A ratio, atrial reverse velocity, LAV 26 E/A ratio (≥1.00), AR velocity (≤25 cm/s), and LA volume (≥107 mL) are significantly associated with stroke Shih et al.18 79 Permanent non valvular AF patients Stroke LA peak strain and LA strain rate during reservoir and conduct, e′ velocity, E/e′. 20 LA peak strain and strain rate during reservoir are independently associated with stroke at multivariate analysis Abbreviations as in Table 1.
Table 2Cross-sectional studies assessing the association of echocardiographic parameters with prevalence of ischaemic stroke/systemic thromboembolism
. No. of patients . Patients enrolled . Event assessed . Echo parameters tested . No. of events . Results . Kim et al.15 1098 Paroxysmal non valvular AF patients undergoing catheter ablation Stroke/TIA LAD, LVMi, LV EF, transmitral E velocity, e′ velocity, s′ velocity, E/e′ 114 LAD, e′ velocity, s′ velocity and E/e′ associated with stroke/TIA at univariate analyses Only E/e′ associated with stroke/TIA at multivariate analysis after controlling for clinical risk factors Lee et al.16 330 Permanent/persistent AF, LV EF > 40%, absence of severe valve disease, congenital heart disease or hypertrophic cardiomyopathy Stroke LAVi, E velocity, e′ velocity, E/e′ 50 E velocity, e′ velocity and E/e′ associated with stroke at univariate analysis. At multivariate regression analysis only E/e′ (odds ratio = 1.21, P = 0.002) associated with stroke Obokata et al.17 285 Non valvular AF patients admitted for acute stroke/embolism (N = 82) and AF control patients without stroke (n = 203) Stroke/ embolism LAVi (max), LAVi (min), LA emptying fraction, LA strain 82 LA strain independently associated with stroke/embolism at multivariate analysis Sakabe et al.19 76 Paroxysmal non-valvular AF with no risk factors or only mild hypertension Stroke E/A ratio, atrial reverse velocity, LAV 26 E/A ratio (≥1.00), AR velocity (≤25 cm/s), and LA volume (≥107 mL) are significantly associated with stroke Shih et al.18 79 Permanent non valvular AF patients Stroke LA peak strain and LA strain rate during reservoir and conduct, e′ velocity, E/e′. 20 LA peak strain and strain rate during reservoir are independently associated with stroke at multivariate analysis . No. of patients . Patients enrolled . Event assessed . Echo parameters tested . No. of events . Results . Kim et al.15 1098 Paroxysmal non valvular AF patients undergoing catheter ablation Stroke/TIA LAD, LVMi, LV EF, transmitral E velocity, e′ velocity, s′ velocity, E/e′ 114 LAD, e′ velocity, s′ velocity and E/e′ associated with stroke/TIA at univariate analyses Only E/e′ associated with stroke/TIA at multivariate analysis after controlling for clinical risk factors Lee et al.16 330 Permanent/persistent AF, LV EF > 40%, absence of severe valve disease, congenital heart disease or hypertrophic cardiomyopathy Stroke LAVi, E velocity, e′ velocity, E/e′ 50 E velocity, e′ velocity and E/e′ associated with stroke at univariate analysis. At multivariate regression analysis only E/e′ (odds ratio = 1.21, P = 0.002) associated with stroke Obokata et al.17 285 Non valvular AF patients admitted for acute stroke/embolism (N = 82) and AF control patients without stroke (n = 203) Stroke/ embolism LAVi (max), LAVi (min), LA emptying fraction, LA strain 82 LA strain independently associated with stroke/embolism at multivariate analysis Sakabe et al.19 76 Paroxysmal non-valvular AF with no risk factors or only mild hypertension Stroke E/A ratio, atrial reverse velocity, LAV 26 E/A ratio (≥1.00), AR velocity (≤25 cm/s), and LA volume (≥107 mL) are significantly associated with stroke Shih et al.18 79 Permanent non valvular AF patients Stroke LA peak strain and LA strain rate during reservoir and conduct, e′ velocity, E/e′. 20 LA peak strain and strain rate during reservoir are independently associated with stroke at multivariate analysis Abbreviations as in Table 1.
Transthoracic echocardiography and surrogate markers of TE events. Some studies highlighted the predictive value of transoesophageal echocardiography (TOE) derived LA cavity and appendage (LAA) abnormalities—such as evidence of thrombi, dense spontaneous echo contrast (SEC), and low-flow velocities (LAA-LF)—in relation with TE events.20–22 Accordingly, TOE parameters have been utilized as surrogate markers of TE events (Table 3). In particular, LA dilatation predicted LAA thrombi, SEC and LF, independent of CHADS2 and CHA2DS2VASc.23,24 In a study on 376 non-valvular AF patients, the addition of LA area (1 point if 24–32.5 cm2, 2 points if > 32.5 cm2) and LV systolic function [1 point if LV ejection fraction (EF) 45–54%, 2 if < 45%) to both CHADS2 and CHA2DS2VASc improved their accuracy in predicting LAA thrombi, LF, and SEC.25 Also LV mass index was found to predict independently LA thrombi formation.26
Table 3Studies assessing the association of echocardiographic parameters with surrogate markers of ischaemic stroke/systemic thromboembolic risk
. No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Results . Doukky et al.24 297 Non valvular AF undergoing TOE and TTE (within 1 year) LAA thrombus at TOE LAVi, e′ velocity, E/e′ LV EF LAVi, e′ velocity and E/e′ are independently associated with the endpoint at multivariate analysis Faustino et al.23 500 AF patients undergoing TTE and TOE before cardioversion LAA thrombus, low LAA velocity, LAA spontaneous echo contrast (SEC) LAD, LAVi, LA area four-chamber index LAVi and LA area four-chamber index are independently associated with the endpoints. Boyd et al.26 165 AF patients undergoing TTE and TOE before cardioversion LAA thrombus LVMi, e′ velocity (TTE), LAA emptying velocity, LA and RA SEC (TOE) LVMi independent predictor of the endpoint at multivariate analysis Providência et al.25 376 Non-valvular AF patients undergoing TOE and TTE LAA thrombus, low LAA velocity, LAASEC LAD, LA area, LV EF Adding LA area and LV global systolic function to CHADS2 and CHA2DS2VASc scores resulted in an improvement of AUC for the prediction of the different endpoints . No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Results . Doukky et al.24 297 Non valvular AF undergoing TOE and TTE (within 1 year) LAA thrombus at TOE LAVi, e′ velocity, E/e′ LV EF LAVi, e′ velocity and E/e′ are independently associated with the endpoint at multivariate analysis Faustino et al.23 500 AF patients undergoing TTE and TOE before cardioversion LAA thrombus, low LAA velocity, LAA spontaneous echo contrast (SEC) LAD, LAVi, LA area four-chamber index LAVi and LA area four-chamber index are independently associated with the endpoints. Boyd et al.26 165 AF patients undergoing TTE and TOE before cardioversion LAA thrombus LVMi, e′ velocity (TTE), LAA emptying velocity, LA and RA SEC (TOE) LVMi independent predictor of the endpoint at multivariate analysis Providência et al.25 376 Non-valvular AF patients undergoing TOE and TTE LAA thrombus, low LAA velocity, LAASEC LAD, LA area, LV EF Adding LA area and LV global systolic function to CHADS2 and CHA2DS2VASc scores resulted in an improvement of AUC for the prediction of the different endpoints Abbreviations as in Table 1.
Table 3Studies assessing the association of echocardiographic parameters with surrogate markers of ischaemic stroke/systemic thromboembolic risk
. No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Results . Doukky et al.24 297 Non valvular AF undergoing TOE and TTE (within 1 year) LAA thrombus at TOE LAVi, e′ velocity, E/e′ LV EF LAVi, e′ velocity and E/e′ are independently associated with the endpoint at multivariate analysis Faustino et al.23 500 AF patients undergoing TTE and TOE before cardioversion LAA thrombus, low LAA velocity, LAA spontaneous echo contrast (SEC) LAD, LAVi, LA area four-chamber index LAVi and LA area four-chamber index are independently associated with the endpoints. Boyd et al.26 165 AF patients undergoing TTE and TOE before cardioversion LAA thrombus LVMi, e′ velocity (TTE), LAA emptying velocity, LA and RA SEC (TOE) LVMi independent predictor of the endpoint at multivariate analysis Providência et al.25 376 Non-valvular AF patients undergoing TOE and TTE LAA thrombus, low LAA velocity, LAASEC LAD, LA area, LV EF Adding LA area and LV global systolic function to CHADS2 and CHA2DS2VASc scores resulted in an improvement of AUC for the prediction of the different endpoints . No. of patients . Patients enrolled . Endpoint . Echo parameters tested . Results . Doukky et al.24 297 Non valvular AF undergoing TOE and TTE (within 1 year) LAA thrombus at TOE LAVi, e′ velocity, E/e′ LV EF LAVi, e′ velocity and E/e′ are independently associated with the endpoint at multivariate analysis Faustino et al.23 500 AF patients undergoing TTE and TOE before cardioversion LAA thrombus, low LAA velocity, LAA spontaneous echo contrast (SEC) LAD, LAVi, LA area four-chamber index LAVi and LA area four-chamber index are independently associated with the endpoints. Boyd et al.26 165 AF patients undergoing TTE and TOE before cardioversion LAA thrombus LVMi, e′ velocity (TTE), LAA emptying velocity, LA and RA SEC (TOE) LVMi independent predictor of the endpoint at multivariate analysis Providência et al.25 376 Non-valvular AF patients undergoing TOE and TTE LAA thrombus, low LAA velocity, LAASEC LAD, LA area, LV EF Adding LA area and LV global systolic function to CHADS2 and CHA2DS2VASc scores resulted in an improvement of AUC for the prediction of the different endpoints Abbreviations as in Table 1.
Rationale for an echo laboratory registry
A lack of definitive data on the power of transthoracic echocardiography in predicting TE events of AF patients remains evident mainly because of the small sample size of the populations assessed. Inhomogeneity of inclusion criteria and covariates, as well as unclear definition of endpoints, limits also the possibility of performing meta-analyses on the existing data. However, some measurements emerge by the above-mentioned studies, in particular taking into account their pathophysiologic meaning in relation with AF development and maintenance. By considering the close association of AF and stroke, all echo-Doppler parameters that have shown to predict AF onset and recurrence could be useful also to predict TE events in this clinical setting.
LA size represents per se a main determinant of AF new onset27–30 and recurrence.31–33 In 881 hypertensive patients with electrocardiogram (ECG)-derived LV hypertrophy (LVH) of the LIFE Study, a greater LA diameter reduction during interventional follow-up was associated with the absence of new-onset AF.34 In the Framingham Heart Study, for every 10 mm increase in LA diameter, the relative risk of stroke was 2.4 in men [95% confidence interval (CI) 1.6–3.7] and 1.4 in women (95% CI 0.9–2.1), even after adjusting for ECG-derived LV mass index.35 Some authors have recently proposed to incorporate LA size in the stroke risk scoring, for a better risk assessment and management of non-valvular AF.36 Additional information could be gained by LA strain, which was found to predict new-onset AF in different clinical settings.37–39
LV diastolic dysfunction and chronic increase of LV filling pressure (LVFP) represent triggers for AF onset, recurrence and permanence. E/e′ ratio predicted the development of AF independent on LA dilation among elderly patients40 and was higher in patients with early onset of AF following cardiac valve surgery.41 The rate of paroxysmal AF increased exponentially with severity of LV diastolic dysfunction after cardiac surgery.42 In 1169 patients with acute myocardial infarction, the risk of new-onset AF was higher in patients with restrictive filling pattern.43
LV hypertrophy (LVH) is a recognized predictor of AF because it induces diastolic dysfunction, LVFP increase, and subsequent LA dilation. In the large population of ARAPACIS Study, patients with non-valvular AF and clear-cut LVH showed higher TE risk.44 In a recent meta-analysis, patients with LVH had three- to four-fold greater odds of developing supraventricular arrhythmias including AF.45 In a restrospective analysis of 171 patients with cryptogenic ischaemic attacks, echocardiographic LVH independently predicted AF detected by Holter ECG.46 In the elderly cohort of the Framingham Study, the hazard ratio (HR) for cerebrovascular events comparing highest to lowest quartile of LV mass index was 2.72 (95% CI 1.39–5.36), after adjusting for several confounders.47
The association of LV systolic dysfunction with AF and TE events is so obvious that LV EF < 40% (as alternative of symptoms/signs of heart failure) is one of the components of CHA2DS2VASc risk score. A vicious circle exists between AF and heart failure and AF predicted new-onset heart failure with preserved EF but not with reduced EF in the Framingham Study.48 Early impairment of LV global longitudinal strain was associated with an increased risk of incident AF in diabetic patients49 and after acute myocardial infarction.50
According to these findings, it is conceivable that the integration of one or more echo Doppler parameter with the available clinical scores could substantially improve TE risk prediction in patients at intermediate risk according to CHA2DS2VASc (CHA2DS2VASc classes 1 if males and 2 if females). Also patients undergoing catheter ablation could benefit from this approach. In this setting of patients, guidelines recommend prosecution of OAC if at high risk, regardless of the success of the procedure, without any definite evidence. Indeed, the German Ablation Registry identified hypertensive heart disease (LVH, LV diastolic or systolic dysfunction) as an independent predictor of periprocedural ischaemic stroke.51
Figure 1 illustrates the key parameters that could be incorporated in TE predicting scores. Among these parameters, left atrial volume index (LAVi)52 and E/e′ ratio53 have the advantage of being more reproducible than LV mass and LV EF54,55 and could be therefore particularly promoted.
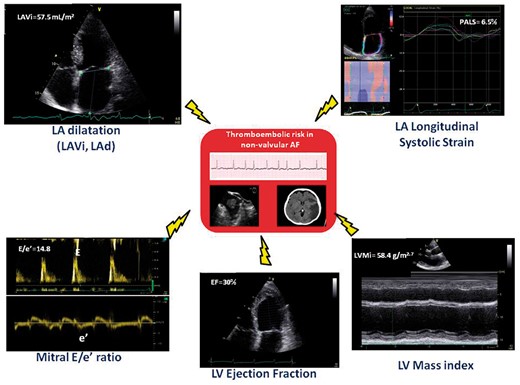
Key echo-Doppler parameters of potential additive value for thromboembolic risk prediction. LA, left atrial; LAd, left atrial diameter; LAVi, left atrial volume index; LV, left ventricular.
Design of the EACVI AFib Echo Europe Registry
EACVI AFib Echo Europe Registry has been designed as an observational, cross-sectional study, with the aim of evaluating the relationship of echocardiographic parameters of LA dimension and function, LV geometry, LV systolic and diastolic function with clinical TE and bleeding risk profile as assessed using CHA2DS2VASc and HASBLED scores.
Secondary objectives of the registry will be:
To evaluate the echocardiographic phenotype of patients with paroxysmal, persistent and permanent AF;
To evaluate the echocardiographic phenotype of patients at intermediate TE risk (CHA2DS2VASc = 1 in men or 2 if women).
The AFib Echo Europe Registry will be a multicentre registry involving 20 reference European echocardiographic laboratories, chosen among the accredited echo labs under the guidance of European National Societies and Working Groups, along a period of 6 months.
Protocol of AFib Echo Europe Registry
All consecutive patients with non-valvular permanent, persistent or paroxysmal AF having a heart rate between 60 and 100 bpm undergoing a transthoracic echocardiographic examination will be recruited in the echo labs participating the Registry during the period of 6 months. Informed consent to the enrolment in the study will be obtained. Exclusion criteria will be previous catheter or surgical ablation, LAA occlusion, cardiac surgery or percutaneous non-coronary interventional procedures, moderate-to-severe mitral and aortic valve stenosis, severe mitral and aortic valve regurgitation, mitral and aortic prosthesis, sepsis and poor acoustic window.
Anthropometric parameters (age, gender, height, and weight), blood pressure, and heart rate will be recorded. A detailed medical history and physical examination will be obtained and data included. Components of CHA2DS2VASC and HAS-BLED scores will be evaluated and recorded. Data on cardiac therapy including also OAC will be also collected.
Scores
CHA2DS2VASc and HAS-BLED scores will be determined after collecting anamnestic and demographic data on individual patients, according to standardized procedures.56,57
Doppler echo parameters
2D, colour, and Doppler echocardiography will include evaluation and recording of the following parameters:
‘LV geometry and systolic function’, according to the European Association of Cardiovascular Imaging (EACVI)/American Society of Echocardiography (ASE) recommendations for chamber quantification58: LV end-diastolic and end-systolic diameters, LV mass index and relative wall thickness, LV end-diastolic, LV end-systolic volumes and EF (Simpson biplane), LV global longitudinal strain (GLS) using 2D speckle-tracking echocardiography.
‘LV diastolic function’, according to the ASE/EACVI recommendations59: pulsed Doppler transmitral inflow pattern parameters: peak E velocity, A velocity, E/A ratio and E velocity deceleration time, pulsed tissue Doppler measurements of septal and lateral mitral annulus with measurement of average (between septal and lateral) e′ velocity, a′ velocity and s′ velocity, and E/e′ ratio. Pulmonary arterial systolic pressure will be estimated from maximal gradient of tricuspid regurgitation (if present) + estimation of right atrial pressure by quantitation of inferior vena cava size and respiratory collapse.
‘LA evaluation’59,60: LA anteroposterior diameter of LA, LA volume (biplane area–length method) index, LA emptying index (by subtracting LA end-diastolic volume from LA end-systolic volume), and LA ejection fraction [(LAEF = LA end-diastolic volume − LA end-systolic volume)/LA end-diastolic volume× 100], LA expansion index (LAFI = LAEF×LVOT-VTI/LAESVI, where LVOT-VTI is velocity time index of LV outflow tract and LAESVI is LA end-systolic volume index), LA peak longitudinal systolic strain (PALS) using speckle-tracking echocardiography, averaged between four-chamber and two-chamber apical views (optional parameter), will be determined.
All the reported echo-Doppler measurements will be averaged from three cardiac cycles in patients in sinus rhythm and from five cardiac cycles during AF.
In addition, evidence and degree of mitral, aortic, and tricuspid stenosis/regurgitation will be reported according to the most recent EACVI recommendation.61,62 Among the enrolled patients, those with a clinical indication for TOE will undergo evaluation of LAA appendage: evidence of LAA thrombi, dense spontaneous echo contrast, and LAA velocities measurement with pulsed-wave Doppler (average of inwards and outwards velocities from three cardiac cycles in patients in sinus rhythm and from five cardiac cycles during AF) will be recorded by this technique.
Quality control
Each peripheral echocardiography laboratory will follow the standard operational procedures recommended by the principal investigator (PI) and the steering committee.
A brief tutorial will be created to explain how to perform all the measures required for the registry in a standardized manner, in agreement with the most recent EACVI recommendations.58–62
The PI will plan to ensure the quality control of echocardiograms performed in peripheral echocardiographic laboratories and optimize reading procedures to limit the inter- and intra-observer variability of measurements. The reproducibility among the laboratories involved will be checked (Bland and Altman test; interclass correlation coefficient, inter-rater agreement: Kappa) on a data set of 15 echocardiographic examinations provided by the PI and the steering committee. Continuous connections will be kept between both PI and steering committee during overall registry duration period.
Ethical committee
The AFib Echo Europe Registry will be developed according to the common rules for research in human subjects. Privacy protection with regard to processing of individual data will be ensured. The ethical committees of the Institutions involved will approve the project. All patients will have to give their written informed consent.
The sample size
According to the previous results derived from a preliminary registry data collected in the Echo Laboratory of Federico II University Hospital, Naples, Italy (n = 200), the expected standard deviation for indexed LA volume and LA strain would be 15.6 mL/m2 and 10.9%, respectively. Using a CI at 95% and a margin at 2.5%, a sample size of 1000 patients would be acceptable. In addition, this sample size will be able to appropriately answer to the secondary objective of the study with high statistical power. This sample size will also allow further statistical multivariate adjustment aiming to identify whether LA parameters are independent determinants of CHA2DS2VASc score. However, missing data can be expected and their management should be planned. In this regard, 17% of LA strain was not available, requiring us to increase the sample size to 1204 patients.
Expectations of the registry
We expect to collect data on the echocardiographic phenotype of patients with any kind of AF in a panorama of reference European echo labs involved in the Registry. The large data set we expect to accumulate will be in particular useful to test the level of agreement of different echocardiographic measurements with the available TE and bleeding risk scores. It is expected that the additional usefulness of echocardiographic examination could emerge to create future projects for stratifying prognosis and improving management in categories of AF patients.
Steering committee of EACVI AFib Echo Registry
Maurizio Galderisi (PI, Chair), Erwan Donal (Vice Chair), Julien Magne (responsible for data management and statistical analyses), Francesco Lo Iudice, Eustachio Agricola, Leyla Elif Sade, Matteo Cameli, Ehud Schwammenthal, Nuno Cardim, Bernard Cosyns, Andreas Hagendorff, Alexander N. Neskovic, Josè Luis Zamorano, Patrizio Lancellotti, Gilbert Habib, Thor Edvardsen, and Bogdan A. Popescu.
Conflict of interest: None declared.
References
Author notes
Reviewer panel: Liza Thomas, Sydney, Australia.



