-
PDF
- Split View
-
Views
-
Cite
Cite
François Regoli, Francesco Faletra, Serena Marcon, Laura Anna Leo, Maria Cristina Dequarti, Maria Luce Caputo, Giulio Conte, Tiziano Moccetti, Angelo Auricchio, Anatomic characterization of cavotricuspid isthmus by 3D transesophageal echocardiography in patients undergoing radiofrequency ablation of typical atrial flutter, European Heart Journal - Cardiovascular Imaging, Volume 19, Issue 1, January 2018, Pages 84–91, https://doi.org/10.1093/ehjci/jew336
Close - Share Icon Share
Abstract
Radiofrequency ablation (RFA) is the treatment of choice of cavotricuspid isthmus (CTI)-dependent atrial flutter. Procedural time is highly variable due to anatomical structures. This study aimed to characterize CTI anatomy by transesophageal 3D echocardiography imaging (3D-TEE) to identify anatomic structures related to longer ablation time.
Thirty-one consecutive patients (mean age 67.3 ± 11.5 years, 22 males) underwent CTI-ablation procedure. Before ablation, TEE was performed and 3D-TEE images were acquired to evaluate CTI anatomy qualitatively as well as perform measures of CTI morphological features. The electrophysiologist performing RFA was blinded to 3D-TEE data. Bidirectional block of CTI was achieved in all patients without procedural complications after a median ablation time of 11 (IQR 7–14) min. Patients with RFA time ≥11 min (Group 2) presented lower left ventricular ejection fraction (51.1 ± 17.0 vs. 59.5 ± 6.6%, P < 0.010), a larger left atrium (46.2 ± 8.4 vs. 39.9 ± 9.4 mm, P < 0.058), and, more frequently, a right atrial pouch (12/16 patients vs. 4/15, P = 0.012) compared with patients with RFA time < 11 min (Group 1); CTI pouch was significantly deeper in Group 2 compared with Group 1: telediastolic (TD) pouch depth was 10.4 ± 4.5 vs. 6.3 ± 1.5 mm (P = 0.003) and telesystolic (TS) depth 12.8 ± 4.4 vs. 7.0 ± 1.4 mm (P < 0.001), respectively. TD isthmus length, prominent pectinate muscle, and presence of an Eustachian ridge (ER) did not differ between the two groups.
Routine pre-procedural 3D-TEE imaging is extremely helpful in qualitative and quantitative evaluation of CTI anatomy in patients undergoing RFA for symptomatic typical atrial flutter. Detection of a deep right atrial pouch was found to be associated with significantly prolonged CTI ablation time to achieve bidirectional block.
Introduction
Radiofrequency ablation (RFA) of the cavo-tricuspid isthmus (CTI) is a widely applied, highly successful non-pharmacological therapy for typical atrial flutter. It is well-recognized, however, that varying anatomical and morphological aspects of the CTI may at times render conduction block across the CTI through catheter ablation difficult to achieve. Several reports on procedural data of CTI ablation have shown a high degree of variability in procedural and fluoroscopy use times1 and that CTI anatomy influences procedural data. Systematic patient-specific pre-procedural evaluation of CTI anatomy is not routinely performed before RFA of typical atrial flutter ablation.
Studies using different representation techniques have been utilized, including fluoroscopic angiography,2,3 intra-cardiac echocardiography (ICE),4 2D transthoracic echocardiography (2D-TTE),5 real-time 3D transesophageal echocardiography (3D-TEE),1 and even magnetic resonance imaging of the heart.6 These studies have consistently demonstrated that particular anatomic features such as prominent Eustachian ridge (ER) and valve (EV), a long isthmus, and the presence of a deep sub-Eustachian pouch, are associated with difficult CTI RFA.1–5
TEE is often used before CTI ablation mainly to exclude atrial thrombus and is, therefore, already part of the workflow of a patient undergoing RFA procedure for typical atrial flutter. In spite of this, evaluation of CTI using 3D-TEE imaging modality is not routinely performed, and thus far there has been no systematic attempt to quantitatively define anatomical structures associated with prolonged ablation time. 3D-TEE offers images of unprecedented quality.7,8 by allowing visualization of CTI morphology and anatomy in great detail, thus making quantification of different atrial structures possible.
The objective of the present study was to characterize CTI anatomy using 3D-TEE imaging modality and to identify and quantify CTI morphological aspects that are associated with prolonged ablation time.
Methods
Patient population
This was a single-center, prospective, observational study which included 31 consecutive patients presenting electrocardiographic documented typical atrial flutter undergoing CTI ablation at the Fondazione Cardiocentro Ticino (Lugano, Switzerland). In all of these patients, TEE (for exclusion of atrial thrombus) was successfully performed. All patients gave written and oral consent for TEE and the RFA procedure.
3D-TEE imaging
TEE was performed by an experienced echocardiographist in all patients before the RF procedure. 3D-TEE images were acquired to evaluate CTI anatomy (Figure 1), using commercially available fully sample matrix array TEE transducer and ultrasound system (X7—2T live 3D TEE transducer, iE33; Philips Medical Systems, Andover, MA, USA). Adequate adjustments of the image and rotation allowed to evaluate the length of the CTI isthmus as well as various CTI anatomic details.
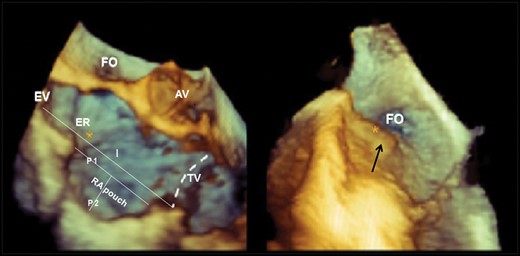
Three-dimensional transesophageal echocardiography (3D-TEE) image of the cavo-tricuspid isthmus (CTI). The CTI is delimited anteriorly by the atrio-ventricular right atrial (RA) groove (dashed line). Main CTI anatomic constituents and the qualitative and quantitative evaluations considered are presented and include Eustachian valve (EV), Eustachian ridge (ER), pectinate muscle (black arrow right panel), isthmus tele-diastolic and tele-systolic lengths (I), and RA pouch tele-diastolic and tele-systolic lengths (P1) and depths (P2). RA Pouch is considered a profound depression of the central CTI (left panel). The right panel focuses on a very prominent muscular irregularity, pectinate muscle (black arrow), usually observed at the level of the infero-lateral CTI. AV, aortic valve; FO, foramen ovale; TV, tricuspid annulus.
Definitions and measurements of the anatomic structures forming the CTI
The CTI is delimited posteriorly by the EV that composes the anterior portion of the inferior vena cava (IVC) ostium; anteriorly to the EV and prominent in its septal aspect, the ER is a more or less prominent muscular band which inserts into the posterior septum and continues obliquely into the superior septum between the foramen ovale and the ostium of the coronary sinus (CS) (Figure 1). Towards the tricuspid valve annulus, below the inferior border of the CS ostium, a semilunar membraneous valve, the Tebesian valve, is frequently associated with a small localized depression called the sub-Tebesian recess. A prominent Eustachian pouch determined by upward folding of the posterior RA endocardium determines a deep concave depression of the whole central isthmus. Moreover, the central isthmus may present irregularities (pectinate muscle) determined by muscular continuity of the crista terminalis which extends across to reach the central isthmus. The lateral CTI is simply the anatomic extension of the crista terminalis to the bottom free wall of the RA composed usually of prominent irregular muscular tissue ramifications known as pectinate muscle. By Pouch is intended an extensive depression of the central isthmus usually associated with an EV; this structure is also known as the sub-Eustachian pouch. Septal recess refers to a localized small septal depression below the ostium of the CS, also referred to as sub-Tebesian recess.
Measures (Figure 1) of anatomic structures were performed off-line using dedicated software (QLAB 10; Philips Medical Systems, Andover, MA, USA) based on 3DTEE acquired images using mainly frontal and sagittal views of the RA. The length of CTI was measured as the linear distance between the tricuspid annulus and the end of the right atrial myocardial sleeve in both atrial telesystolic (TS) and telediastolic (TD) phases. Other quantitative measurements included TD and TS depths of central CTI concavities, when these were present. EV and ER were estimated as either prominent, moderate or unremarkable based on the extent of tissue protrusion forming the anterior aspect of the IVC ostium for the EV, and the degree of muscular protrusion towards the inter-atrial septum for the ER. Final, encroachment of pectinate muscles into the central CTI was estimated as abundant, moderate extent, or absent according to the entity of muscular irregularities extending to this anatomical area. Total duration of the 3D TEE acquisition and of the subsequent off-line post-processing was registered.
The reproducibility of echocardiographic analysis was performed on 10 3D-TEE datasets, randomly selected and analysed again three months later by the original observer and by another observer, blinded to the results of the previous analysis.
CTI ablation procedure
Anti-arrhythmic drugs were discontinued at least five half-lives before the procedure, and oral anti-coagulation therapy was stopped at 24–48 h before the procedure when the patient was treated with non-vitamin K antagonist (VKA) and at least 72 h before in patients under VKA. The interventional electrophysiologist was blinded to the 3D TEE findings. The RFA has been described in detail elsewhere.1 Briefly, RFA was performed using conventional 8 mm ablation catheter, with fixed ablation settings (Power: 80 W; Temperature: 65 °C). RFA was always initiated by applying a fluoroscopic left anterior oblique 6 o’clock view CTI line, and then, if this did not yield bidirectional conduction block, a CTI infero-lateral line was performed. The endpoint of the ablation procedure was the achievement of complete bidirectional CTI block persisting for a waiting period of 30 min.
Statistics
Continuous variables are expressed as the SD around the mean or as median with inter-quartile range (IQR) between brackets. Normally distributed data were compared using independent Student’s t-tests. Categorical values and relative frequencies were compared using Fisher exact test. All tests were two-tailed.
Intra-observer and inter-observer correlations of quantitative CTI measurements and agreement of CTI qualitative evaluations were assessed. Intra-observer and inter-observer correlation of various measurements (CTI lengths, lengths, and depths of pouch) were assessed using Spearman’s correlation coefficient (R). For qualitative evaluation of anatomical features such as the EV, kappa statistics were used, and the level of agreement was interpreted as follows: 0–0.2= poor to slight, 0.21–0.4 = fair, 0.41–0.6 = moderate, 0.61–0.8 = substantial, and 0.81–1.0 = nearly perfect. Stata version 11 (StataCorp LP, College Station, TX, USA) was used for computation. A P value <0.05 was considered statistically significant for all tests.
Results
The baseline demographic characteristics of the study population are presented in Table 1.
| . | Patients (n = 31) . |
|---|---|
| Male sex | 22 |
| Age, years | 67.3 ± 11.5 |
| Hypertension | 18 |
| Coronary artery disease | 9 |
| Valve disease | 5 |
| Other CV disease | 6 |
| ‘Lone’ atrial flutter | 16 |
| COPD | 3 |
| Left atrial diameter, mm | 43.3 ± 9.3 |
| LVEF, % | 55.0 ± 13.7 |
| Paroxysmal atrial flutter | 11 |
| Persistent atrial flutter | 20 |
| History of atrial fibrillation | 16 |
| Medication | |
| Amiodarone | 9 |
| Sotalol | 2 |
| Ic anti-arrhythmic drugs | 3 |
| Beta-blocke r | 20 |
| Digitalis | 2 |
| Ca channel blocker | 2 |
| ACEI/AT blocker | 13 |
| Diuretic | 9 |
| Statin | 11 |
| Oral anti-coagulation | 26 |
| Anti-platelet agent | 15 |
| . | Patients (n = 31) . |
|---|---|
| Male sex | 22 |
| Age, years | 67.3 ± 11.5 |
| Hypertension | 18 |
| Coronary artery disease | 9 |
| Valve disease | 5 |
| Other CV disease | 6 |
| ‘Lone’ atrial flutter | 16 |
| COPD | 3 |
| Left atrial diameter, mm | 43.3 ± 9.3 |
| LVEF, % | 55.0 ± 13.7 |
| Paroxysmal atrial flutter | 11 |
| Persistent atrial flutter | 20 |
| History of atrial fibrillation | 16 |
| Medication | |
| Amiodarone | 9 |
| Sotalol | 2 |
| Ic anti-arrhythmic drugs | 3 |
| Beta-blocke r | 20 |
| Digitalis | 2 |
| Ca channel blocker | 2 |
| ACEI/AT blocker | 13 |
| Diuretic | 9 |
| Statin | 11 |
| Oral anti-coagulation | 26 |
| Anti-platelet agent | 15 |
COPD, chronic obstructive pulmonary disease; CV, cardiovascular; LVEF, left ventricular ejection fraction.
| . | Patients (n = 31) . |
|---|---|
| Male sex | 22 |
| Age, years | 67.3 ± 11.5 |
| Hypertension | 18 |
| Coronary artery disease | 9 |
| Valve disease | 5 |
| Other CV disease | 6 |
| ‘Lone’ atrial flutter | 16 |
| COPD | 3 |
| Left atrial diameter, mm | 43.3 ± 9.3 |
| LVEF, % | 55.0 ± 13.7 |
| Paroxysmal atrial flutter | 11 |
| Persistent atrial flutter | 20 |
| History of atrial fibrillation | 16 |
| Medication | |
| Amiodarone | 9 |
| Sotalol | 2 |
| Ic anti-arrhythmic drugs | 3 |
| Beta-blocke r | 20 |
| Digitalis | 2 |
| Ca channel blocker | 2 |
| ACEI/AT blocker | 13 |
| Diuretic | 9 |
| Statin | 11 |
| Oral anti-coagulation | 26 |
| Anti-platelet agent | 15 |
| . | Patients (n = 31) . |
|---|---|
| Male sex | 22 |
| Age, years | 67.3 ± 11.5 |
| Hypertension | 18 |
| Coronary artery disease | 9 |
| Valve disease | 5 |
| Other CV disease | 6 |
| ‘Lone’ atrial flutter | 16 |
| COPD | 3 |
| Left atrial diameter, mm | 43.3 ± 9.3 |
| LVEF, % | 55.0 ± 13.7 |
| Paroxysmal atrial flutter | 11 |
| Persistent atrial flutter | 20 |
| History of atrial fibrillation | 16 |
| Medication | |
| Amiodarone | 9 |
| Sotalol | 2 |
| Ic anti-arrhythmic drugs | 3 |
| Beta-blocke r | 20 |
| Digitalis | 2 |
| Ca channel blocker | 2 |
| ACEI/AT blocker | 13 |
| Diuretic | 9 |
| Statin | 11 |
| Oral anti-coagulation | 26 |
| Anti-platelet agent | 15 |
COPD, chronic obstructive pulmonary disease; CV, cardiovascular; LVEF, left ventricular ejection fraction.
Feasibility and data reproducibility of 3D TEE imaging of CTI
3DTEE CTI imaging was considered of good quality in most cases (84%). Mean TEE total duration was 15.2 ± 3.7 min, including 3D image acquisition and CTI qualitative assessment; mean off-line post-processing time for measurements was 5.1 ± 1.9 min (quantitative assessment).
Intra-observer and inter-observer correlation was very high (r > 0.7) for all quantitative measures performed. Intra-observer and inter-observer agreement was excellent with k values >0.80 (P < 0.05) for each evaluated CTI anatomic feature.
3D-TEE characterization of CTI
The median length of CTI was 41 mm (IQR 38–49 mm); in 15 patients (48%) the CTI length was above the median value, and it was, therefore, labelled as ‘long CTI’. RA pouch is defined as a concave depression of the CTI with TS depth ≥8 mm, based on the inferior SD margin around mean TS depth of the whole cohort (mean TS depth for the whole cohort: 12.2 ± 4.5 mm). In 24 patients (77%) there was one or more anatomical feature: marked visible EV (10 patients, 32%), large ER (11 patients, 35%), presence of pouch (16 patients, 52%), or pectinate muscles (14 patients, 45%). Eleven patients (35%) presented a combination of three or more of the above anatomical features.
Anatomic and morphological CTI determinants of prolonged ablation time
Bidirectional block was achieved in all patients. No procedural complications occurred. Median ablation time was 11 min (IQR: 7–14 min). RF time ≥ 11 min to achieve bidirectional CTI block was required in 16 patients (Group 2); mean ablation time in Group 2 was 14.3 ± 8.9 min compared with 6.8 ± 1.9 min for Group 1 (P = 0.003) (Table 2).
Comparison of 3D transesophageal echocardiographic and ablation radiofrequency data between patients with a straightforward CTI ablation (Group 1) compared with patients with difficult (Group 2) CTI ablation
| Variables . | Group 1 (n = 15) (<11 min) . | Group 2 (n = 16) (≥11 min) . | P value . |
|---|---|---|---|
| 3D-TEE data | |||
| CTI TD length (mm) | 40.0±8.8 | 43.1±4.9 | 0.362 |
| CTI TS length (mm) | 32.1±8.5 | 38.2±5.4 | 0.032 |
| Pouch | 2 | 12 | 0.001 |
| TD length | 20.0±8.0 | 25.1±5.5 | 0.060 |
| TS length | 18.0±7.0 | 20.5±7.5 | 0.370 |
| TD depth | 6.3±1.5 | 10.4±4.5 | 0.003 |
| TS depth | 8.3±1.5 | 12.9±4.6 | 0.001 |
| Pectinate muscles | |||
| Smooth/mild | 10 | 6 | 0.128 |
| Moderate/very prominent | 5 | 4 | |
| Eustachian valve | 2 | 8 | 0.054 |
| Eustachian ridge | 3 | 8 | 0.135 |
| Pouch and other RA feature | 0.023 | ||
| 0–1 | 11 | 5 | |
| ≥2 | 4 | 11 | |
| LA diameter | 39.9±9.4 | 46.2±8.4 | 0.058 |
| Left ventricular ejection fraction (%) | 51.1±17.0 | 59.5±6.6 | 0.010 |
| Radiofrequency ablation data | |||
| n. applications | 6.3±1.5 | 13.4±8.7 | 0.005 |
| RFA time (min) | 6.8±1.9 | 14.3±8.9 | 0.003 |
| Variables . | Group 1 (n = 15) (<11 min) . | Group 2 (n = 16) (≥11 min) . | P value . |
|---|---|---|---|
| 3D-TEE data | |||
| CTI TD length (mm) | 40.0±8.8 | 43.1±4.9 | 0.362 |
| CTI TS length (mm) | 32.1±8.5 | 38.2±5.4 | 0.032 |
| Pouch | 2 | 12 | 0.001 |
| TD length | 20.0±8.0 | 25.1±5.5 | 0.060 |
| TS length | 18.0±7.0 | 20.5±7.5 | 0.370 |
| TD depth | 6.3±1.5 | 10.4±4.5 | 0.003 |
| TS depth | 8.3±1.5 | 12.9±4.6 | 0.001 |
| Pectinate muscles | |||
| Smooth/mild | 10 | 6 | 0.128 |
| Moderate/very prominent | 5 | 4 | |
| Eustachian valve | 2 | 8 | 0.054 |
| Eustachian ridge | 3 | 8 | 0.135 |
| Pouch and other RA feature | 0.023 | ||
| 0–1 | 11 | 5 | |
| ≥2 | 4 | 11 | |
| LA diameter | 39.9±9.4 | 46.2±8.4 | 0.058 |
| Left ventricular ejection fraction (%) | 51.1±17.0 | 59.5±6.6 | 0.010 |
| Radiofrequency ablation data | |||
| n. applications | 6.3±1.5 | 13.4±8.7 | 0.005 |
| RFA time (min) | 6.8±1.9 | 14.3±8.9 | 0.003 |
P values in bold (P<0.05) are considered significant for any test. 3D-TEE, 3D transesophageal echocardiography; CTI TD, cavotricuspid isthmus tele-diastolic; CTI TS, cavotricuspid isthmus tele-systolic; LA, left atrium; RA, right atrium; RFA, radiofrequency ablation; TD, tele-diastolic; TS, tele-systolic.
Comparison of 3D transesophageal echocardiographic and ablation radiofrequency data between patients with a straightforward CTI ablation (Group 1) compared with patients with difficult (Group 2) CTI ablation
| Variables . | Group 1 (n = 15) (<11 min) . | Group 2 (n = 16) (≥11 min) . | P value . |
|---|---|---|---|
| 3D-TEE data | |||
| CTI TD length (mm) | 40.0±8.8 | 43.1±4.9 | 0.362 |
| CTI TS length (mm) | 32.1±8.5 | 38.2±5.4 | 0.032 |
| Pouch | 2 | 12 | 0.001 |
| TD length | 20.0±8.0 | 25.1±5.5 | 0.060 |
| TS length | 18.0±7.0 | 20.5±7.5 | 0.370 |
| TD depth | 6.3±1.5 | 10.4±4.5 | 0.003 |
| TS depth | 8.3±1.5 | 12.9±4.6 | 0.001 |
| Pectinate muscles | |||
| Smooth/mild | 10 | 6 | 0.128 |
| Moderate/very prominent | 5 | 4 | |
| Eustachian valve | 2 | 8 | 0.054 |
| Eustachian ridge | 3 | 8 | 0.135 |
| Pouch and other RA feature | 0.023 | ||
| 0–1 | 11 | 5 | |
| ≥2 | 4 | 11 | |
| LA diameter | 39.9±9.4 | 46.2±8.4 | 0.058 |
| Left ventricular ejection fraction (%) | 51.1±17.0 | 59.5±6.6 | 0.010 |
| Radiofrequency ablation data | |||
| n. applications | 6.3±1.5 | 13.4±8.7 | 0.005 |
| RFA time (min) | 6.8±1.9 | 14.3±8.9 | 0.003 |
| Variables . | Group 1 (n = 15) (<11 min) . | Group 2 (n = 16) (≥11 min) . | P value . |
|---|---|---|---|
| 3D-TEE data | |||
| CTI TD length (mm) | 40.0±8.8 | 43.1±4.9 | 0.362 |
| CTI TS length (mm) | 32.1±8.5 | 38.2±5.4 | 0.032 |
| Pouch | 2 | 12 | 0.001 |
| TD length | 20.0±8.0 | 25.1±5.5 | 0.060 |
| TS length | 18.0±7.0 | 20.5±7.5 | 0.370 |
| TD depth | 6.3±1.5 | 10.4±4.5 | 0.003 |
| TS depth | 8.3±1.5 | 12.9±4.6 | 0.001 |
| Pectinate muscles | |||
| Smooth/mild | 10 | 6 | 0.128 |
| Moderate/very prominent | 5 | 4 | |
| Eustachian valve | 2 | 8 | 0.054 |
| Eustachian ridge | 3 | 8 | 0.135 |
| Pouch and other RA feature | 0.023 | ||
| 0–1 | 11 | 5 | |
| ≥2 | 4 | 11 | |
| LA diameter | 39.9±9.4 | 46.2±8.4 | 0.058 |
| Left ventricular ejection fraction (%) | 51.1±17.0 | 59.5±6.6 | 0.010 |
| Radiofrequency ablation data | |||
| n. applications | 6.3±1.5 | 13.4±8.7 | 0.005 |
| RFA time (min) | 6.8±1.9 | 14.3±8.9 | 0.003 |
P values in bold (P<0.05) are considered significant for any test. 3D-TEE, 3D transesophageal echocardiography; CTI TD, cavotricuspid isthmus tele-diastolic; CTI TS, cavotricuspid isthmus tele-systolic; LA, left atrium; RA, right atrium; RFA, radiofrequency ablation; TD, tele-diastolic; TS, tele-systolic.
The two groups differed in terms of LVEF and left atrial size (Table 2, Figure 2A) as well as proportion of prescribed medication; more patients in Group 2 (n = 10 patients) were treated with ACE inhibitor/sartan (compared with only three in Group 1, P = 0.03).
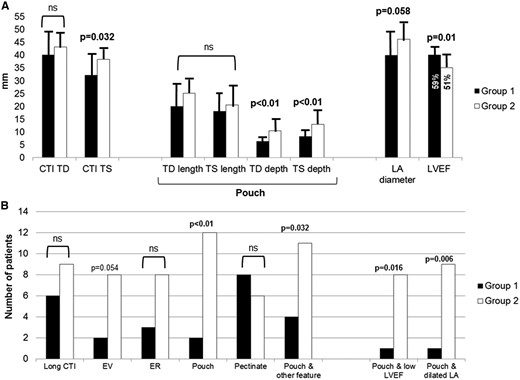
(A) Comparative measures with corresponding SD of CTI length and pouch size between patients with straightforward (Group 1, black columns) and patients with prolonged radiofrequency ablation (RFA) time (Group 2, white columns). (B) Comparative qualitative assessment of prominent anatomic CTI features, and corresponding structural and morphological associations, between patients with straightforward (Group 1, black columns) and those with prolonged RFA time (Group 2, white columns). ‘Pouch’ is considered a profound CTI depression with TS depth ≥8 mm, based on the inferior SD margin around the mean TS depth value of the whole cohort. Long CTI is considered Telediastolic CTI length above the median length >41 mm; low LVEF is considered below the median value of 55%; dilated LA is considered median LA antero-posterior telediastolic diameter >43 mm. CTI, cavotricuspid isthmus; EV, Eustachian valve; ER, Eustachian ridge; LVEF, left ventricular ejection fraction; LA, left atrium; TD, tele-diastolic; TS, tele-systolic.
More complex CTI anatomy (Table 2, Figure 2A and B) was detected by 3D-TEE in Group 2 patients: these patients more frequently presented a prominent EV, a longer isthmus and, most importantly, deep RA pouch. TD pouch length (25.1 ± 5.8 mm vs. 0.0 ± 8.0 mm, P = 0.060) and, especially, TD and TS pouch depths (TD depth: 10.4 ± 4.5 mm vs. 6.3 ± 1.5 mm, P = 0.003; TS depth: 12.8 ± 4.4 mm vs. 7.0 ± 1.4 mm, P < 0.001) were significantly greater in Group 2 (Table 2, Figure 2A).
Figure 2B presents the comparative distribution of morphological and structural features of the CTI in the two groups. In Group 2 patients, RA pouch was often anatomically bound to another prominent feature, such as EV; furthermore, RA pouch was significantly associated with LA enlargement (P = 0.006) and/or depressed LVEF (P = 0.016). Conversely, combination of prominent pectinate muscle encroaching into the inferior CTI from the crista terminalis and RA pouch was an infrequent anatomical finding (only 2/31 patients, 6%). Other features, namely presence of prominent pectinate muscle as well as presence of a prominent ER did not differ significantly between the two groups (Table 2, Figure 2B).
Discussion
The present study showed the importance of anatomic characterization of CTI by 3DTEE imaging in detecting anatomical variations and pathological changes of the morphology of the right atrium which are associated with prolonged ablation time in patients with symptomatic typical atrial flutter. The main CTI anatomic determinant causing lengthening of RFA time was the presence of RA pouch (defined as an extensive CTI depression with TS depth ≥8 mm), often associated with a prominent EV or ER. This particular anatomic variant was found to be more prevalent in patients with enlarged LA and lower LVEF, thus possibly indicating remodeling of CTI in patients with structural heart disease.
Other imaging modalities for CTI anatomy characterization to guide RF ablation
Several studies have characterized CTI anatomy using RA angiography.2,3 This modality, implicating the use of fluoroscopy and contrast dye injection, allows to define the anatomical contours of the right atrium and detected highly variable CTI length (ranging from 30 to 40 mm), shape (straight, concave, pouch), and IVC angle in patients with typical flutter. Interestingly, using this imaging modality Heidbüchel et al.2 reported a similar incidence of sub-Eustachian pouch as that in our series (47%) with a mean depth which was reported to be around half (4 mm) compared with our series averaging 8–9 mm in depth, according to the cardiac phase in which the structure was measured. While these differences are in part related to different imaging modalities, our 3DTEE data, in line with what has consistently been shown by CTI angiography studies, confirm that a concave-shaped CTI due to the presence of a deep sub-Eustachian pouch represents one of the most important anatomic determinants of prolonged ablation time in the treatment of CTI-dependent atrial flutter. Through the use of ICE, requiring invasive access and post-processing to obtain 3D imaging, Scaglione et al.4 reported that complex CTI anatomy, described as ‘hills and valleys’ anatomy of the CTI, was associated with a greater number of RFA applications. This morphological CTI variant was present in 30% of our patient cohort. However, evaluation of CTI structures like the ER and pouch using ICE are difficult because the plane visualized is oblique rather than perpendicular, it is, therefore, not possible to measure cross-sectional anatomy precisely.4
Chen et al.,5 using a modified long axis 2D-TTE view also reported that the presence of a long isthmus, extensive ER, presence of RA pouch as well as presence of tricuspid regurgitation were linked to more difficult ablation. Of these features, extensive ER proved to be the only independent predictor of difficult ablation in their series. Despite some differences between 3D-TEE and 2D-TTE, a similar incidence of long isthmus was found in our experience (35%) compared with that of Chen and colleagues (30%). The modest difference in the proportion of long isthmus may be related to the fact that 3D-TEE has a higher resolution compared with transthoracic 2D modality, thus allowing to better define specific morphological features particularly in the posterior portion of the CTI. The ER, a posterior paraseptal muscular band, should be distinguished from the membraneous EV which spans anteriorly across from the IVC ostium from septum to the postero-inferior RA free wall. In the study by Chen et al.,5 no mention is, however, made of the EV and therefore the presence of a sub-Eustachian pouch is likely undervalued, and is rather considered simply a concave-shaped CTI. These aspects outline once again how anatomic interpretation is highly influenced by the quality and resolution of the imaging modality utilized.
Our observations are confirmatory of previous studies but significantly expand current knowledge. In fact, for the first time we have been able to provide quantitative measurements which may guide typical common atrial flutter ablation procedural planning. The higher definition and resolution of 3D-TEE imaging allows to reach such an anatomic detail that approaches dissected anatomic specimen. In line with detailed autoptic reports of the CTI in a general population,9 3D-TEE imaged the co-existence, in the same subject, of multiple CTI anatomic features, including possible co-existence of EV, ER, as well as RA pouch. As described in autoptic specimen, the presence of a deep pouch is bound to valvular structures, namely the Eustachian, the Tebesian, and tricuspid valves.9 While the first two valves were usually imaged with 3DTEE using sagittal and frontal views, the particular location of the Tebesian valve in the inter-atrial septum in proximity to the inferior septal portion of the tricuspid valve, required some effort to view. For these reasons, systematic assessment of the Tebesian valve was not performed in this study. Worthy of note, our 3DTEE data confirm another aspect of CTI autoptic data: an inverse relation was found between the presence of prominent pectinate muscle and that of a deep RA pouch. In our experience, these CTI features co-existed in only 2 out of the 31 patients (6%) considered.
In fact, as suggested from anatomic interpretation of CTI from autopsies on large number of subjects,9 3D-TEE anatomic characterization of the CTI indicates that the anatomical CTI variant presenting a deep RA pouch, represents the most challenging anatomic variant to treat with RFA (Figure 3, left panel). Another variant developed by continuation of crista terminalis tissue with the final muscular ramifications (pectinate muscle) that encroach into the CTI causing the inferior isthmus to be irregular, but generally flat, without valvular structures, and rather short in length (Figure 3, right panel), was usually associated with a straightforward RFA procedure.
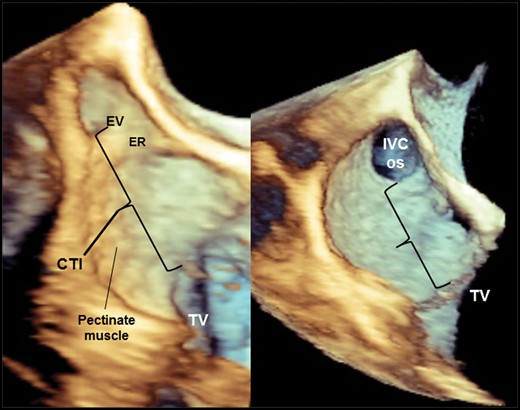
These two 3D TEE images depict structural and morphological heterogeneity of the CTI. Left panel: anatomically complex CTI presenting all features requiring longer ablation time to achieve bidirectional block. Right panel: regular flat-shaped CTI without the presence of depressions; there are only modest surface irregularities determined by pectinate muscles which represent the continuation of muscular ramifications from the crista terminalis. IVS os, inferior vena cava ostium; ER, Eustachian ridge; EV, Eustachian valve; TV, tricuspid valve.
Finally, the finding that a deep RA pouch is often associated to an enlarged LA and reduced LVEF, suggests that concave deformation of the CTI may be an anatomical expression of RA structural remodeling.
Clinical implications of 3D TEE imaging of anatomical structures for CTI ablation
Longer RF time as well as greater number of RF lesions performed, both significantly higher in Group 2, imply sub-optimal ablation catheter contact and stability to deliver RF energy to the tissue. As previously expressed by Asivartham,10 specific anatomic features of the CTI may influence RF energy delivery in different ways. A long CTI isthmus, implies limited contact pressure to the distal portion of the CTI. The presence of a prominent ER has the effect of rotating the ablation catheter tip (and therefore contact force) away from the RA septum and towards the lateral CTI. Clearly if this latter aspect occurs in presence of a deep pouch, achieving adequate contact with the floor of the pouch may be very challenging and therefore achievement of CTI bidirectional block difficult. Figure 4 presents how integration of pre-procedural 3D-TEE quantitative and qualitative information on CTI anatomy may guide RFA approach (Figure 4). Being technically prepared before the ablation procedure to confront a challenging CTI anatomy, by targeting immediately a lateral CTI line with the use of a transvenous long sheath, for instance (Figure 4), may potentially reduce RF delivery time, thus preventing possible rare, but severe complications, such as RA perforation with tamponade,11,12 atrio-ventricular block,13 or even right coronary artery lesion.14 Furthermore, precise pre-procedural knowledge of CTI anatomy by 3D TEE imaging may potentially reduce exposure to ionizing radiation during X-ray fluoroscopy to both patient and operator thus reducing long-term risk of biological effect.15
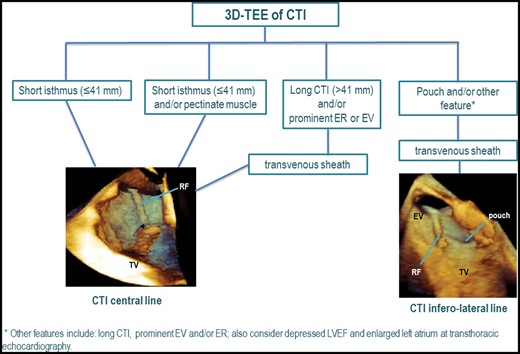
3D-TEE-guided ablation of CTI in typical atrial flutter. A flow-chart is proposed on how to adapt RFA approach in patients with typical atrial flutter according to CTI anatomy. The 3D-TEE images are modified from the contribution by Regoli et al.1 3D-TEE, three-dimensional transesophageal echocardiography; CTI, cavo-tricuspid isthmus; ER, Eustachian ridge; EV, Eustachian valve; LVEF, left ventricular ejection fraction; RF, radiofrequency ablation catheter; TV, tricuspid annulus.
Study limitations
This study was a single-center study with a relatively small patient population. Even though 3D-TEE imaging of CTI was straightforward, achieved in patients without entailing an important lengthening of the TEE examination, the laboratory performing this examination possesses a high-level of expertise in this imaging technique. 3D TEE imaging was presented in great detail in several previous publications.7,8 The main challenge, therefore, is represented by the accurate and quick acquisition of 3D TEE images to correctly evaluate CTI anatomy. The anatomic data were distinguished in ‘qualitative’ and ‘quantitative’, the ‘qualitative’ data being quicker to acquire not requiring off-line post-processing. In the experience of our laboratory involved in the training of fellows in cardiac imaging, the skills required to make these acquisitions are within the reach of every experienced cardiac imaging specialist.
CTI anatomic determinants of difficult ablation were identified by retrospective analysis once the ablation procedure was completed. Although the electrophysiologist was ‘blinded’ to CTI anatomic data, other patient- and operator-specific aspects may have influenced RF ablation time in a non-quantifiable way. The results of our study clearly need to be validated in a prospective and controlled scheme comparing pre-procedural 3D-TEE-guided approach with the conventional one.
Conclusions
This study demonstrated that routine pre-procedural 3D TEE imaging is extremely helpful in the qualitative and quantitative evaluation of CTI anatomy. Specific anatomic features, such as deep right atrial pouch, were found to be associated with significantly prolonged CTI ablation time to achieve bidirectional block in patients undergoing RFA for symptomatic typical atrial flutter. This cardiac imaging modality is already well-integrated in the current workflow of patients undergoing RFA of CTI, and may, therefore, provide valuable information to the electrophysiologist for more efficient peri-procedural planning.
Conflict of interest: FR: consultant to Daiichi Sankyo, Medtronic, LivaNova, and Bayer; AA: consultant to LivaNova, DC Devices, Medtronic, EBR Systems, Biosense Webster, Biologics Delivery Systems Group, Bristol-Myers Squibb, Infobionics, Resmed, and Leadexx. Receives speaker fees from Biotronik GmBH, Medtronic, Resmed, and Sorin Group. Other authors: no disclosures.



