-
PDF
- Split View
-
Views
-
Cite
Cite
Frederik H Verbrugge, Navigating the risks in acute heart failure, European Heart Journal. Acute Cardiovascular Care, Volume 9, Issue 5, 1 August 2020, Pages 372–374, https://doi.org/10.1177/2048872620941790
Close - Share Icon Share
Poor visibility, icebergs, strong currents and wind speeds reaching 125 km per hour render Drake Passage – the body of water between South America’s Cape Horn and Antarctica – one of the most treacherous waters in the world. How striking is the resemblance with acute heart failure (AHF), in which patient and clinician navigate together in order to avoid the many pitfalls that would lead to undesirable outcomes. The course has proved too difficult for most of our randomised clinical trials and studies that have evaluated a long list of treatment strategies, pharmacological agents and devices. As a result, clinical outcomes in AHF remain dismal, with recent European registry data demonstrating an inhospital mortality of 5.5%, 1-year mortality of 26.7% and 1-year rehospitalisation rate of 44.4%.1 This issue of European Heart Journal: Acute Cardiovasc Care therefore focuses on a better understanding of these risks, which may hopefully result in a more successful future approach.
Most patients with AHF present through the emergency department, which offers a first opportunity for risk stratification and management. The study group on acute heart failure of the Acute Cardiovascular Care Association presents a systematic review of available risk scores that have been validated to predict short-term mortality and adverse events in AHF.2 What immediately strikes the eye is that even the best performers among those scores only narrowly beat a C-statistic of 0.8 that is considered a requirement for a strong model. This is in spite of weighing up a considerable number of variables (⩾10 for most scores), including laboratory results and most often the electrocardiogram. It just illustrates how challenging the job is to predict the individual trajectory of a patient with AHF, who present with very heterogeneous phenotypes. Future approaches, with artificial intelligence analysing big data contained within the electronic medical record, may improve these efforts, but efficient and accurate data input, connectivity between data-containing media and protection of sensible information are barriers that will need to be overcome.
Better characterisation of distinct AHF phenotypes may offer an opportunity to provide more individually tailored therapy, as all-comer strategies have too often failed. Biomarkers could be used for this purpose. Cancer antigen 125 (CA 125) is such an emerging biomarker with potential usefulness in AHF. Originally conceptualised as a tumour marker for ovarian cancer, CA 125 is synthesised by mesothelial cells in response to inflammation or increased hydrostatic venous pressure. Consequently, it also directly relates to congestion. Miñana et al. present an interesting study that compares AHF phenotypes identified by CA 125 versus the more routinely assessed N-terminal pro-hormone of B-type natriuretic peptide (NT-proBNP).3 CA 125 levels were most strongly associated with present pleural effusion and tricuspid valve regurgitation severity, which together explained approximately 60% of its variability but less than 2% of that from NT-proBNP. Hence, it is tempting to speculate that CA 125 levels reflect the volumetric component of congestion, while NT-proBNP corresponds better with pressure overload. This could have important implications to guide diuretic therapy, which is further supported by a small, open-label study from the same group of investigators.4 Nevertheless, further confirmation is required before routine implementation can be recommended.
The role of vasodilator therapy in AHF remains unclear. From a pathophysiological perspective, unloading the ventricle with vasodilators primarily benefits patients with low stroke volume yet relatively maintained blood pressure because of intense vasoconstriction. This corresponds phenotypically to a patient with reduced ejection fraction and low pulse pressure. In this population, alleviating afterload by reducing vascular resistance through vasodilators improves forward flow and organ perfusion, with a neutral effect on mean arterial pressure as stroke volume increases. Counterintuitively, those who benefit often have low systolic blood pressure (<120 mmHg).5 The study by Shiraishi et al. focused on the other side of the blood pressure spectrum in AHF, showing that vasodilators reduced inhospital mortality in patients with systolic blood pressure greater than 180 mmHg.6 It would be interesting to learn whether such individuals might have more intrinsic vascular/endothelial dysfunction or volume redistribution rather than overload per se. One wonders about their CA 125 levels!
AHF is not only a problem of the heart. Llauger et al. remind us that kidney dysfunction is frequent and is associated with worse outcomes.7 Anaemia, another prevalent comorbidity in AHF, adds independent risk.7 Although a rising serum creatinine is common during decongestive treatment, this should generally not preclude the pursuit of complete decongestion, neither lead to withdrawal of disease-modifying drugs.8 Indeed, serum creatinine has many limitations as a surrogate for kidney dysfunction, which explains why novel biomarkers – more specific for tubular injury and structural nephron damage – are explored. N-acetyl-β-D-glucosamidase is among the most prognostically relevant of those and might predict the pace of future renal function decline.9 Funabashi et al. confirm its prognostic utility in the largest AHF sample studied up till now.10
Preservation of organ viability and tissue integrity is an important goal in AHF. Cellular damage and necrosis lead to the release of intracellular contents into the systemic circulation. Krychtiuk et al. demonstrate how mitochondrial deoxyribonucleic acid (mtDNA) levels intriguingly track the severity of chronic heart failure as assessed by New York Heart Association functional class.11 In addition, much higher mtDNA levels were observed in AHF, which was independently associated with mortality. The authors postulate that mtDNA should be further investigated as a biomarker for tissue integrity in AHF and might even be directly implied in its pathophysiology through pro-inflammatory effects. This legitimate hypothesis deserves further study.
The last study by Nakano et al. is a gem, because of its simplicity and the exciting hypotheses generated. The authors found a robust relationship between increased base excess (>2 mmol/l) and long-term mortality in AHF.12 Despite wide availability and easy acquisition through arterial blood gas analysis, base excess had never been studied properly before in the context of AHF. While its value as a risk marker is interesting on its own, the more captivating question is why there was a relationship with outcome. Increased base excess may indicate appropriate metabolic compensation for respiratory acidosis, but partial pressure carbon dioxide (pCO2) levels were not terribly elevated and the prevalence of chronic obstructive pulmonary disease was low (<10%). Moreover, the interaction between pCO2 and base excess to predict mortality was not statistically significant. Thus, respiratory comorbidity was not likely to have explained the observed relationship. Instead, an increased strong ion difference – mainly due to lower serum chloride levels – was the major determinant of high base excess. Chloride depletion is an important cause of diuretic resistance that is known to be associated with poor clinical outcomes in AHF.13 Importantly, loop and thiazide-type diuretics that constitute the default decongestive strategy may worsen hypochloraemia. In contrast, proximally working diuretics such as acetazolamide and sodium-glucose cotransporter-2 inhibitors boost natriuresis while preserving chloride. The base excess may therefore inform clinicians on appropriate diuretic management. Whether such guided interventions actually improve clinical outcomes requires further study.
While the treatment of patients with AHF remains a formidable challenge, this issue of European Heart Journal: Acute Cardiovasc Care hopefully provides a compass to sail a more steady future course. The accompanying Figure 1 to this editorial summarises a systematic approach that focuses on how findings discussed may find their way into clinical practice.
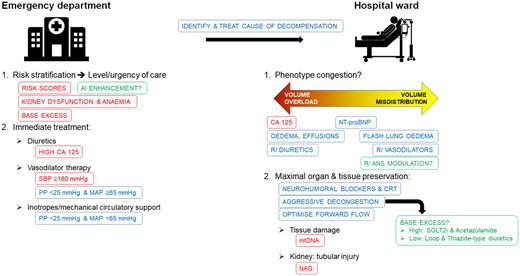
A systematic approach to acute heart failure: contemporary strategies in blue, strategies supported by evidence in this issue of European Heart Journal: Acute Cardiovasc Care in red, and potential future enhancements in green.
AI: artificial intelligence; ANS: autonomic nerve system; CA 125: cancer antigen 125; CRT: cardiac resynchronisation therapy; MAP: mean arterial blood pressure; mtDNA: mitochondrial desoxyribonucleic acid; NAG: N-acetyl-β-D-glucosamidase; NT-proBNP: N-terminal of pro-hormone B-type natriuretic peptide; PP: pulse pressure; SBP: systolic blood pressure; SGLT2is: sodium-glucose co-transporter 2 inhibitors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: FHV is supported by a fellowship of the Belgian American Educational Foundation (BAEF) and by the special research fund (BOF) of Hasselt University (BOF19PD04).




Comments