-
PDF
- Split View
-
Views
-
Cite
Cite
Matilde Winther-Jensen, Jesper Kjaergaard, Christian Hassager, Lars Køber, Freddy Lippert, Helle Søholm, Cancer is not associated with higher short or long-term mortality after successful resuscitation from out-of-hospital cardiac arrest when adjusting for prognostic factors, European Heart Journal. Acute Cardiovascular Care, Volume 9, Issue 4_suppl, 1 November 2020, Pages S184–S192, https://doi.org/10.1177/2048872618794090
Close - Share Icon Share
As the prevalence of malignancies in the general population increases, the odds of an out-of-hospital cardiac arrest (OHCA) patient having a history of cancer likewise increases, and the impact on post-cardiac arrest care and mortality is not well known. We aimed to investigate 30-day and 1-year mortality after successful resuscitation in patients with cancer prior to OHCA compared with OHCA patients without a previous cancer diagnosis.
A cohort of 993 consecutive OHCA patients with successful resuscitation during 2007–2011 was included. Vital status was obtained from the Danish Civil Register, and cancer diagnoses from the Danish National Patient Register dating back to 1994. Primary endpoints were 30-day, 1-year and long-term mortality (no cancer: mean 811 days; cancer: mean 406 days), analysed by Cox regression. Functional status assessed by cerebral performance category at discharge and use of post-resuscitation care were secondary endpoints.
A total of 119 patients (12%) were diagnosed with cancer prior to OHCA. Mortality was higher in patients with cancer (30-day 69% vs. 58%, P=0.01); however, after adjustment for prognostic factors cancer was no longer associated with higher mortality (hazard ratio (HR)30 days 0.98, 95% confidence interval (CI) 0.76–1.27, P=0.88; HR1 year 0.99, 95% CI 0.78–1.27, P=0.96 HRend of follow-up 0.95, 95% CI 0.75–1.20, P=0.67). Favourable cerebral performance category scores in patients alive at discharge did not differ (cerebral performance category 1 or 2 n=310 (84%) vs. n=31 (84%), P=1).
Cancer prior to OHCA was not associated with higher mortality in patients successfully resuscitated from OHCA when adjusting for confounders. Cancer prior to OHCA should be used with caution when performing prognostication after OHCA.
Introduction
Out-of-hospital cardiac arrest (OHCA) carries a high mortality risk, even though survival has increased markedly in previous years.1,,–4 This may be due to increased focus on bystander cardiopulmonary resuscitation1,5 as well as improved post-resuscitation care, including early revascularisation, target temperature management (TTM) and neurological assessment.6,7 However, 30-day survival is still only approximately 10%,1 thereby making OHCA one of the most fatal medical events.
As the frequency of OHCA increases with age,8,9 and the incidence of patients surviving cancer increases,10,11 the number of patients previously diagnosed with cancer who experience OHCA is likely to increase. At the same time, some cancer treatments have cardiovascular side-effects, which has led to increasing focus on cardiovascular prognosis in cancer patients.12 Thus the need for prognostication of patients diagnosed with cancer and surviving OHCA is also likely to increase. Higher 1 or 3-year mortality in OHCA patients with a history of cancer has been found,13,14 but the definition of cancer varies, as some only consider active malignancies defined as requiring active treatment.14
Previous studies examining cardiac arrest and cancer have often been studies of inhospital cardiac arrest (IHCA). However, hospitalised patients are more likely to be severely ill at the time of arrest,15,–17 even though patients with IHCA may be resuscitated successfully.18 It is likely necessary to distinguish between IHCA and OHCA patients with cancer prior to arrest as well as between different cancer types, and to consider the length of time elapsed since the cancer diagnosis.
We hypothesised that cancer patients would have a worse outcome than patients without cancer and thus aimed to: (a) assess the influence of cancer prior to OHCA on 30-day and 1-year mortality as well as mortality at end of follow up; (b) assess whether mortality in OHCA patients with active (diagnosis <5 years prior to OHCA) and previous (diagnosis ⩾5 years prior to OHCA) cancer differed from OHCA patients without cancer; (c) determine if the use of post-resuscitation care differed between patients with and without cancer; and finally (d) determine if functional status at hospital discharge differed between patients with and without cancer.
Methods
Study population
Between 2007 and 2011, patients aged 18 years and over with OHCA from all causes, who achieved successful resuscitation and were attended by the emergency medical services (EMS) were included consecutively (Figure 1).
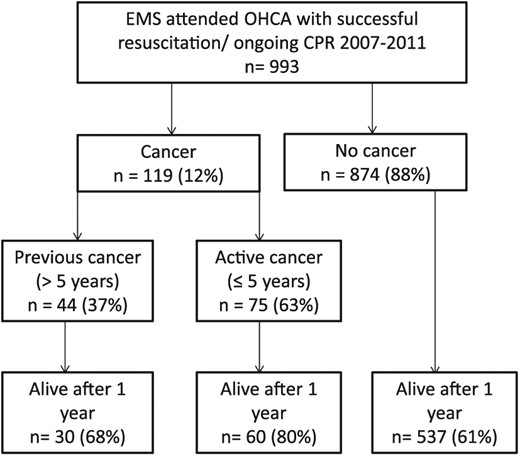
Flow chart of included patients. All patients successfully resuscitated from OHCA and attended by the EMS in the greater Copenhagen area between 2007 and 2011.
Abbreviations: EMS: Emergency Medical Services, CPR: Cardiopulmonary resuscitation, OHCA: Out-of-hospital cardiac arrest.
The study area, the 675 km2 (260 square mile) Copenhagen area, was inhabited by approximately 1.2 million people during the study period. The Copenhagen EMS were organised with an ambulance with basic life support equipment and a separate vehicle operated by an attending physician and a paramedic. After each dispatch, the EMS personnel completed an Utstein-style form,19 in which prehospital details on the OHCA event were entered.
Outcome
Mortality data
Vital status was obtained from the Danish Civil Register. We used 30-day and 1-year mortality and mortality at the end of follow-up in March 2016.
Functional outcome
Neurological outcome was assessed at hospital discharge by cerebral performance category (CPC), a scale ranging from 1 to 5.19 A score of 1–2 was defined as a favourable outcome, 3–4 was defined as unfavourable and 5 was assigned to dead patients. Patient charts were reviewed in order to assign CPC scores; this was done blinded to previous assessment and interobserver reliability was excellent (kappaweighted 1.0).
Comorbidity
Previous hospital diagnoses were obtained from the Danish National Patient Register dating back to 1994 until 2011 by using the unique personal registration number, which is used in all contacts with the healthcare system. Diagnoses prior to OHCA were used to identify cancer patients, as patients with International Classification of Disease (ICD) 10 diagnoses C00–D48, except D10–D36 (benign neoplasms), were considered to have cancer. A and B diagnoses were both considered, and diagnoses 5 years or more relative to OHCA were categorised as ‘previous cancer’, while diagnoses less than 5 years from the OHCA were categorised as ‘active cancer’. The authors acknowledge that some cancers still have high mortality even 5 years after diagnosis, but as 5 years is the time span most often used for cancer-specific survival, we chose this interval. Analysis of the association between cancer and mortality was performed with and without considering basal and squamous cell skin cancer, which is often considered a less malignant type of cancer due to its inability to metastasise.
The Charlson comorbidity index (CCI) was used to assess the comorbidity burden in all patients. The CCI is a validated and weighted index commonly used to assess short-term mortality.20,21 Diagnoses for CCI were acquired from patient charts and the Danish National Patient Register. We excluded cancer diagnoses (equalling CCI groups ‘any tumour’, ‘leukaemia’, ‘lymphoma’, ‘metastatic solid tumour’) from the CCI index, in order to be able to assess the non-cancer comorbidity burden.
Post-resuscitation care
We assessed the use of post-resuscitation care factors: the number of patients with admission to intensive care units (ICUs), use of TTM, use of an implantable cardioverter defibrillator (ICD) or pacemaker before hospital discharge, performance of two-dimensional echocardiography, acute coronary angiography (CAG) (<24 hours), revascularisation (percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)) in patients undergoing CAG, and whether the patient consulted a cardiologist during the hospital admission. Patients with termination of active therapy in the emergency department were excluded from the post-resuscitation care analyses, as these patients were not able to undergo the mentioned procedures.
Statistics
Baseline differences between patients with and without cancer were tested by t-test for numerical variables and by chi-square test for categorical variables, using Monte Carlo estimation on encountering counts of less than 5 for categorical variables (including neurological outcome as measured by the CPC scale). Monte Carlo simulation can be used when test assumptions are not met and computes P values based on randomly generated samples with a reference distribution. Univariate and multiple logistic regression analyses were used in testing influence on favourable versus unfavourable neurological outcome with adjustment for age, sex, cancer, primary rhythm, witnessed arrest, CCI of 3 or greater and time to return of spontaneous circulation (ROSC), presented as odds ratios (ORs) with 95% confidence intervals (CIs). We also assessed the use of post-resuscitation care factors, in logistic regression with the use of procedure (e.g. CAG) as outcome, adjusting for cancer, sex, age at arrest (5-year increase), primary rhythm, witnessed arrest, CCI of 3 or greater and time to ROSC (5-minute increase). This was only done in patients surviving emergency department admission, and revascularisation was only assessed in patients undergoing CAG.
Kaplan–Meier plots were used to assess differences in 30-day and 1-year mortality, and multiple Cox regression analyses was used to estimate the influence on mortality between OHCA patients with and without cancer, presented as hazard ratios (HRs) with 95% CI, adjusting for age, sex, primary rhythm, witnessed arrest, CCI of 3 or greater and time to ROSC. In addition, we assessed mortality at end of follow-up (no cancer: mean 811 days; cancer: mean 406 days). We also assessed whether mortality differed between patients with active and previous cancer in multivariate Cox regression analysis, and whether the results would differ if basal and squamous cell skin cancer was not considered cancer (including these patients in the non-cancer group).
Results
We identified 993 consecutive patients successfully resuscitated from OHCA, of which 119 patients (12%) had a pre-OHCA cancer diagnosis (Figure 1). The different cancer diagnoses are depicted in Figure 2. The most common diagnoses were breast cancer (18%), basal and squamous cell skin cancer (12%), prostate cancer (11%), pulmonary/pleural cancer (10%) and gastrointestinal cancer (10%).
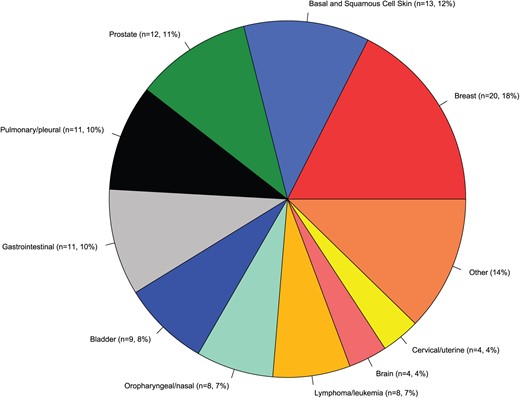
Most common cancer diagnoses in successfully resuscitated OHCA patients with cancer diagnosis prior to OHCA.
Differences between patients with and without cancer
Patients with and without cancer did not differ with regard to prehospital circumstances, except for shockable primary rhythm, which was more common in patients without cancer (43% in cancer patients vs. 54% in patients without cancer, Table 1). With regard to demographics, age and comorbidity burden as measured by CCI differed between patients with and without cancer (73 vs. 65 years, P<0.001; CCI of 3 or greater: 36% vs. 16%, P<0.001). Although not reaching statistical significance, cancer patients seemed more often to have suicide as the cause of OHCA (2% vs. 0.5%, P=0.16).
Demographic, prehospital and post-resuscitation care characteristics in patients with OHCA with and without cancer prior to OHCA.
| . | Cancer n=119 . | No cancer n=874 . | P value . |
|---|---|---|---|
| Demography | |||
| Male sex (n (%)) | 78 (65%) | 628 (72%) | 0.19 |
| Age, years (median (Q1–Q3)) | 73 (63–82) | 65 (54–75) | <0.001 |
| Non-cancer comorbidity burden (CCI of 3 or greater)a | 43 (36%) | 136 (16%) | <0.001 |
| Prehospital factors | |||
| Primary rhythmb | |||
| VF/VT (n (%)) | 51 (43%) | 468 (54%) | 0.04 |
| PEA (n (%)) | 31 (26%) | 167 (19%) | |
| Asystole (n (%)) | 24 (20%) | 183 (21%) | |
| Other/unknown (n (%)) | 13 (11%) | 56 (6%) | |
| OHCA in public (n (%)) | 35 (29%) | 331 (38%) | 0.09 |
| Bystander witnessed OHCA (n (%)) | 103 (86%) | 739 (85%) | 0.70 |
| EMS witnessed arrest (n (%)) | 10 (8%) | 58 (7%) | 0.55 |
| Bystander CPR (n (%)) | 69 (58%) | 486 (56%) | 0.57 |
| Aetiology | |||
| Cardiac (n (%)) | 90 (75%) | 705 (81%) | 0.21 |
| Suicide | 2 (2%) | 4 (0.5%) | 0.16 |
| Drowning | 1 (0.8%) | 3 (0.3%) | 0.43 |
| Pulmonary | 7 (6%) | 41 (5%) | 0.76 |
| Exsanguination | 1 (0.8%) | 6 (0.7%) | 1 |
| Suffocation/strangulation | 4 (3%) | 17 (2%) | 0.47 |
| Trauma | 1 (0.8%) | 15 (2%) | 0.69 |
| STEMI | 19 (16%) | 215 (25%) | 0.05 |
| NSTEMI | 20 (17%) | 130 (15%) | 0.65 |
| Time to ROSC (minutes) (median (Q1–Q3)) | 14 (9–21) | 15 (9–22) | 0.44 |
| Time to EMS arrival (minutes) (median (Q1–Q3)) | 7 (5–9) | 7 (5–9) | 0.76 |
| Post-resuscitation care | |||
| Termination of inhospital care due to comorbidity | 35 (29%) | 90 (10%) | <0.001 |
| Admitted to ICU (n (%))c | 81 (68%) | 671 (77%) | 0.046 |
| Target temperature management (n (%))c | 37 (31%) | 395 (45%) | <0.001 |
| Awake post-OHCA (n (%)) | 18 (15%) | 93 (11%) | 0.16 |
| Consulted with a cardiologist during hospital admission (n (%))c | 83 (70%) | 657 (75%) | 1 |
| ICD or pacemaker (n (%))c | 9 (8%) | 146 (17%) | 0.02 |
| Echocardiography performed during hospital admission (n (%))c | 61 (51%) | 558 (64%) | 0.01 |
| CAG | 32 (27%) | 351 (40%) | 0.009 |
| CAG within 24 hours post-OHCA | 27 (26%) | 266 (30%) | 0.13 |
| Revascularisation (n (%))d | 17 (53%) | 228 (65%) | 0.25 |
| PCI | 15 (13%) | 199 (23%) | 0.38 |
| CABG | 2 (2%) | 37 (4%) | 0.56 |
| LVEF at hospital discharge | |||
| <35% (low) | 26 (22%) | 235 (27%) | |
| 35–50% (moderately decreased) | 16 (13%) | 163 (19%) | 0.82 |
| >50% (preserved) | 22 (18%) | 179 (21%) |
| . | Cancer n=119 . | No cancer n=874 . | P value . |
|---|---|---|---|
| Demography | |||
| Male sex (n (%)) | 78 (65%) | 628 (72%) | 0.19 |
| Age, years (median (Q1–Q3)) | 73 (63–82) | 65 (54–75) | <0.001 |
| Non-cancer comorbidity burden (CCI of 3 or greater)a | 43 (36%) | 136 (16%) | <0.001 |
| Prehospital factors | |||
| Primary rhythmb | |||
| VF/VT (n (%)) | 51 (43%) | 468 (54%) | 0.04 |
| PEA (n (%)) | 31 (26%) | 167 (19%) | |
| Asystole (n (%)) | 24 (20%) | 183 (21%) | |
| Other/unknown (n (%)) | 13 (11%) | 56 (6%) | |
| OHCA in public (n (%)) | 35 (29%) | 331 (38%) | 0.09 |
| Bystander witnessed OHCA (n (%)) | 103 (86%) | 739 (85%) | 0.70 |
| EMS witnessed arrest (n (%)) | 10 (8%) | 58 (7%) | 0.55 |
| Bystander CPR (n (%)) | 69 (58%) | 486 (56%) | 0.57 |
| Aetiology | |||
| Cardiac (n (%)) | 90 (75%) | 705 (81%) | 0.21 |
| Suicide | 2 (2%) | 4 (0.5%) | 0.16 |
| Drowning | 1 (0.8%) | 3 (0.3%) | 0.43 |
| Pulmonary | 7 (6%) | 41 (5%) | 0.76 |
| Exsanguination | 1 (0.8%) | 6 (0.7%) | 1 |
| Suffocation/strangulation | 4 (3%) | 17 (2%) | 0.47 |
| Trauma | 1 (0.8%) | 15 (2%) | 0.69 |
| STEMI | 19 (16%) | 215 (25%) | 0.05 |
| NSTEMI | 20 (17%) | 130 (15%) | 0.65 |
| Time to ROSC (minutes) (median (Q1–Q3)) | 14 (9–21) | 15 (9–22) | 0.44 |
| Time to EMS arrival (minutes) (median (Q1–Q3)) | 7 (5–9) | 7 (5–9) | 0.76 |
| Post-resuscitation care | |||
| Termination of inhospital care due to comorbidity | 35 (29%) | 90 (10%) | <0.001 |
| Admitted to ICU (n (%))c | 81 (68%) | 671 (77%) | 0.046 |
| Target temperature management (n (%))c | 37 (31%) | 395 (45%) | <0.001 |
| Awake post-OHCA (n (%)) | 18 (15%) | 93 (11%) | 0.16 |
| Consulted with a cardiologist during hospital admission (n (%))c | 83 (70%) | 657 (75%) | 1 |
| ICD or pacemaker (n (%))c | 9 (8%) | 146 (17%) | 0.02 |
| Echocardiography performed during hospital admission (n (%))c | 61 (51%) | 558 (64%) | 0.01 |
| CAG | 32 (27%) | 351 (40%) | 0.009 |
| CAG within 24 hours post-OHCA | 27 (26%) | 266 (30%) | 0.13 |
| Revascularisation (n (%))d | 17 (53%) | 228 (65%) | 0.25 |
| PCI | 15 (13%) | 199 (23%) | 0.38 |
| CABG | 2 (2%) | 37 (4%) | 0.56 |
| LVEF at hospital discharge | |||
| <35% (low) | 26 (22%) | 235 (27%) | |
| 35–50% (moderately decreased) | 16 (13%) | 163 (19%) | 0.82 |
| >50% (preserved) | 22 (18%) | 179 (21%) |
Excluded: Previous tumours, previous haematological malignancies, previous metastatic tumours.
Tested as shockable rhythm vs. non-shockable rhythm.
Patients surviving emergency department admission.
Patients surviving emergency department admission and undergoing CAG.
CABG: coronary artery bypass grafting; CCI: Charlson comorbidity index; CPR: cardiopulmonary resuscitation; EMS: emergency medical services; LVEF: left ventricular ejection fraction; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; PEA: pulseless electrical activity; ROSC: return of spontaneous circulation; SD: standard deviation; VF: ventricular fibrillation; VT: ventricular tachycardia; ICU: intensive care unit.
Demographic, prehospital and post-resuscitation care characteristics in patients with OHCA with and without cancer prior to OHCA.
| . | Cancer n=119 . | No cancer n=874 . | P value . |
|---|---|---|---|
| Demography | |||
| Male sex (n (%)) | 78 (65%) | 628 (72%) | 0.19 |
| Age, years (median (Q1–Q3)) | 73 (63–82) | 65 (54–75) | <0.001 |
| Non-cancer comorbidity burden (CCI of 3 or greater)a | 43 (36%) | 136 (16%) | <0.001 |
| Prehospital factors | |||
| Primary rhythmb | |||
| VF/VT (n (%)) | 51 (43%) | 468 (54%) | 0.04 |
| PEA (n (%)) | 31 (26%) | 167 (19%) | |
| Asystole (n (%)) | 24 (20%) | 183 (21%) | |
| Other/unknown (n (%)) | 13 (11%) | 56 (6%) | |
| OHCA in public (n (%)) | 35 (29%) | 331 (38%) | 0.09 |
| Bystander witnessed OHCA (n (%)) | 103 (86%) | 739 (85%) | 0.70 |
| EMS witnessed arrest (n (%)) | 10 (8%) | 58 (7%) | 0.55 |
| Bystander CPR (n (%)) | 69 (58%) | 486 (56%) | 0.57 |
| Aetiology | |||
| Cardiac (n (%)) | 90 (75%) | 705 (81%) | 0.21 |
| Suicide | 2 (2%) | 4 (0.5%) | 0.16 |
| Drowning | 1 (0.8%) | 3 (0.3%) | 0.43 |
| Pulmonary | 7 (6%) | 41 (5%) | 0.76 |
| Exsanguination | 1 (0.8%) | 6 (0.7%) | 1 |
| Suffocation/strangulation | 4 (3%) | 17 (2%) | 0.47 |
| Trauma | 1 (0.8%) | 15 (2%) | 0.69 |
| STEMI | 19 (16%) | 215 (25%) | 0.05 |
| NSTEMI | 20 (17%) | 130 (15%) | 0.65 |
| Time to ROSC (minutes) (median (Q1–Q3)) | 14 (9–21) | 15 (9–22) | 0.44 |
| Time to EMS arrival (minutes) (median (Q1–Q3)) | 7 (5–9) | 7 (5–9) | 0.76 |
| Post-resuscitation care | |||
| Termination of inhospital care due to comorbidity | 35 (29%) | 90 (10%) | <0.001 |
| Admitted to ICU (n (%))c | 81 (68%) | 671 (77%) | 0.046 |
| Target temperature management (n (%))c | 37 (31%) | 395 (45%) | <0.001 |
| Awake post-OHCA (n (%)) | 18 (15%) | 93 (11%) | 0.16 |
| Consulted with a cardiologist during hospital admission (n (%))c | 83 (70%) | 657 (75%) | 1 |
| ICD or pacemaker (n (%))c | 9 (8%) | 146 (17%) | 0.02 |
| Echocardiography performed during hospital admission (n (%))c | 61 (51%) | 558 (64%) | 0.01 |
| CAG | 32 (27%) | 351 (40%) | 0.009 |
| CAG within 24 hours post-OHCA | 27 (26%) | 266 (30%) | 0.13 |
| Revascularisation (n (%))d | 17 (53%) | 228 (65%) | 0.25 |
| PCI | 15 (13%) | 199 (23%) | 0.38 |
| CABG | 2 (2%) | 37 (4%) | 0.56 |
| LVEF at hospital discharge | |||
| <35% (low) | 26 (22%) | 235 (27%) | |
| 35–50% (moderately decreased) | 16 (13%) | 163 (19%) | 0.82 |
| >50% (preserved) | 22 (18%) | 179 (21%) |
| . | Cancer n=119 . | No cancer n=874 . | P value . |
|---|---|---|---|
| Demography | |||
| Male sex (n (%)) | 78 (65%) | 628 (72%) | 0.19 |
| Age, years (median (Q1–Q3)) | 73 (63–82) | 65 (54–75) | <0.001 |
| Non-cancer comorbidity burden (CCI of 3 or greater)a | 43 (36%) | 136 (16%) | <0.001 |
| Prehospital factors | |||
| Primary rhythmb | |||
| VF/VT (n (%)) | 51 (43%) | 468 (54%) | 0.04 |
| PEA (n (%)) | 31 (26%) | 167 (19%) | |
| Asystole (n (%)) | 24 (20%) | 183 (21%) | |
| Other/unknown (n (%)) | 13 (11%) | 56 (6%) | |
| OHCA in public (n (%)) | 35 (29%) | 331 (38%) | 0.09 |
| Bystander witnessed OHCA (n (%)) | 103 (86%) | 739 (85%) | 0.70 |
| EMS witnessed arrest (n (%)) | 10 (8%) | 58 (7%) | 0.55 |
| Bystander CPR (n (%)) | 69 (58%) | 486 (56%) | 0.57 |
| Aetiology | |||
| Cardiac (n (%)) | 90 (75%) | 705 (81%) | 0.21 |
| Suicide | 2 (2%) | 4 (0.5%) | 0.16 |
| Drowning | 1 (0.8%) | 3 (0.3%) | 0.43 |
| Pulmonary | 7 (6%) | 41 (5%) | 0.76 |
| Exsanguination | 1 (0.8%) | 6 (0.7%) | 1 |
| Suffocation/strangulation | 4 (3%) | 17 (2%) | 0.47 |
| Trauma | 1 (0.8%) | 15 (2%) | 0.69 |
| STEMI | 19 (16%) | 215 (25%) | 0.05 |
| NSTEMI | 20 (17%) | 130 (15%) | 0.65 |
| Time to ROSC (minutes) (median (Q1–Q3)) | 14 (9–21) | 15 (9–22) | 0.44 |
| Time to EMS arrival (minutes) (median (Q1–Q3)) | 7 (5–9) | 7 (5–9) | 0.76 |
| Post-resuscitation care | |||
| Termination of inhospital care due to comorbidity | 35 (29%) | 90 (10%) | <0.001 |
| Admitted to ICU (n (%))c | 81 (68%) | 671 (77%) | 0.046 |
| Target temperature management (n (%))c | 37 (31%) | 395 (45%) | <0.001 |
| Awake post-OHCA (n (%)) | 18 (15%) | 93 (11%) | 0.16 |
| Consulted with a cardiologist during hospital admission (n (%))c | 83 (70%) | 657 (75%) | 1 |
| ICD or pacemaker (n (%))c | 9 (8%) | 146 (17%) | 0.02 |
| Echocardiography performed during hospital admission (n (%))c | 61 (51%) | 558 (64%) | 0.01 |
| CAG | 32 (27%) | 351 (40%) | 0.009 |
| CAG within 24 hours post-OHCA | 27 (26%) | 266 (30%) | 0.13 |
| Revascularisation (n (%))d | 17 (53%) | 228 (65%) | 0.25 |
| PCI | 15 (13%) | 199 (23%) | 0.38 |
| CABG | 2 (2%) | 37 (4%) | 0.56 |
| LVEF at hospital discharge | |||
| <35% (low) | 26 (22%) | 235 (27%) | |
| 35–50% (moderately decreased) | 16 (13%) | 163 (19%) | 0.82 |
| >50% (preserved) | 22 (18%) | 179 (21%) |
Excluded: Previous tumours, previous haematological malignancies, previous metastatic tumours.
Tested as shockable rhythm vs. non-shockable rhythm.
Patients surviving emergency department admission.
Patients surviving emergency department admission and undergoing CAG.
CABG: coronary artery bypass grafting; CCI: Charlson comorbidity index; CPR: cardiopulmonary resuscitation; EMS: emergency medical services; LVEF: left ventricular ejection fraction; OHCA: out-of-hospital cardiac arrest; PCI: percutaneous coronary intervention; PEA: pulseless electrical activity; ROSC: return of spontaneous circulation; SD: standard deviation; VF: ventricular fibrillation; VT: ventricular tachycardia; ICU: intensive care unit.
Post-resuscitation care
More cancer patients had inhospital care terminated due to comorbidity (29% vs. 10%, P<0.001). Fewer patients with cancer were admitted to an ICU (68% vs. 77%, P=0.046) and were treated with TTM compared to patients without cancer (31% vs. 45%, P<0.001), Table 1. There was no difference in patients who were awake after resuscitation and therefore not cooled (15% vs. 11%, P=0.15). There was a lower implantation rate of an ICD or a pacemaker before hospital discharge (8% vs. 17%, P=0.02) and also a lower use of CAG (27% vs. 40%, P=0.009). The incidence of left ventricular ejection fraction less than 35% at hospital discharge did not differ between patients with and without cancer (22% vs. 27%, P=0.82).
By multivariable logistic regression analysis with adjustment for prognostic factors, cancer was found to be associated with lower odds of TTM (OR 0.42, 95% CI 0.22–0.78, P=0.004). There was no difference in the odds of consulting a cardiologist during the hospital admission (OR 1.14, 95% CI 0.48–3.08, P=0.78), for the use of an ICD or pacemaker (O: 0.66, 95% CI 0.29–1.37, P=0.29), acute CAG (OR 0.97, 95% CI 0.56–1.66, P=0.90) nor for revascularisation (PCI or CABG in patients undergoing CAG) (OR 0.76, 95% CI 0.35–1.70, P=0.5).
Mortality
Thirty-day mortality was 69% in successfully resuscitated OHCA patients with cancer and 58% in patients without cancer, Plogrank=0.01. After 1 year, 76% of cancer patients and 61% of patients without cancer were dead, Plogrank=0.002, Figure 3. At the end of follow-up, 87% of patients with cancer and 72% of patients without cancer were dead, P<0.001. The mean total follow-up was 811 (0–3325) days for patients without cancer and 406 (0–3095) days for patients with cancer.
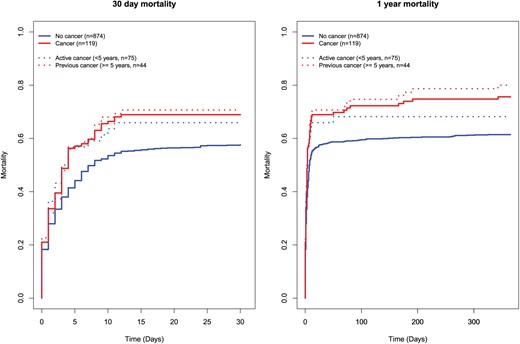
30-day and 1-year mortality in successfully resuscitated patients with and without cancer, and patients with active and previous cancer.
Cancer was associated with higher 30-day mortality in univariate Cox regression analysis (HR 1.35, 95% CI 1.07–1.71, Table 2), but this association was no longer significant after adjusting for known prognostic factors (sex, age, primary rhythm, witnessed arrest, CCI of 3 or greater and time to ROSC) (HR 0.98, 95% CI 0.76–1.27). The same was seen for 1-year mortality and mortality at the end of follow-up as a cancer diagnosis was significantly associated with mortality in univariate analysis, Table 2. When stratifying cancer into active (<5 years prior to OHCA) and previous (⩾5 years prior to OHCA) cancer, there was no differences between patients with active cancer compared to no cancer, and patients with previous cancer compared to no cancer; neither for 30-day, 1-year mortality or mortality at the end of follow-up (30 days: HRactive 1.05, 95% CI 0.77–1.43, HRprevious 0.88, 95% CI 0.58–1.32; 1 year: HRactive 1.11, 95% CI 0.83–1.48, HRprevious 0.82, 95% CI 0.55–1.22, end of follow-up: HRactive 1.11, 95% CI 0.84–1.46, HRprevious 0.86, 95% CI 0.60–1.24).
Factors associated with 30-day and 1-year mortality in patients with and without cancer prior to successful resuscitation from OHCA in univariate and multivariate analyses.
| . | 30-Day mortality . | 1-Year mortality . | End of follow-up (no cancer: mean 811 days; cancer: mean 406 days) . | |||
|---|---|---|---|---|---|---|
| . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . |
| Cancer prior to OHCA | 1.35 (1.07–1.71) | 0.98 (0.76–1.27) | 1.41 (1.13–1.76) | 0.99 (0.78–1.27) | 1.49 (1.21–1.83) | 1.01 (0.80–1.27) |
| Male sex | 0.64 (0.54–0.76) | 0.93 (0.77–1.13) | 0.63 (0.53–0.74) | 0.92 (0.77–1.11) | 0.64 (0.54–0.74) | 0.92 (0.77–1.10) |
| Age (5-year increase) | 1.14 (1.11–1.18) | 1.14 (1.10–1.18) | 1.15 (1.12–1.19) | 1.15 (1.11–1.19) | 1.18 (1.14–1.21) | 1.17 (1.13–1.21) |
| Shockable primary rhythm | 0.34 (0.28–0.40) | 0.31 (0.25–0.37) | 0.33 (0.28–0.39) | 0.32 (0.26–0.38) | 0.36 (0.31–0.41) | 0.35 (0.30–0.42) |
| Bystander witnessed OHCA | 0.69 (0.55–0.86) | 0.78 (0.61–0.997) | 0.68 (0.55–0.84) | 0.76 (0.60–0.96) | 0.69 (0.56–0.84) | 0.72 (0.58–0.90) |
| CCI of 3 or greater (cancer excluded) | 1.6 (1.32–1.95) | 1.32 (1.07–1.64) | 1.72 (1.43–2.08) | 1.42 (1.16–1.74) | 1.91 (1.60–2.27) | 1.59 (1.31–1.92) |
| Time to ROSC (pr. 5 minutes) | 1.08 (1.05–1.11) | 1.14 (1.11–1.18) | 1.07 (1.04–1.10) | 1.13 (1.10–1.17) | 1.05 (1.02–1.08) | 1.11 (1.07–1.14) |
| . | 30-Day mortality . | 1-Year mortality . | End of follow-up (no cancer: mean 811 days; cancer: mean 406 days) . | |||
|---|---|---|---|---|---|---|
| . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . |
| Cancer prior to OHCA | 1.35 (1.07–1.71) | 0.98 (0.76–1.27) | 1.41 (1.13–1.76) | 0.99 (0.78–1.27) | 1.49 (1.21–1.83) | 1.01 (0.80–1.27) |
| Male sex | 0.64 (0.54–0.76) | 0.93 (0.77–1.13) | 0.63 (0.53–0.74) | 0.92 (0.77–1.11) | 0.64 (0.54–0.74) | 0.92 (0.77–1.10) |
| Age (5-year increase) | 1.14 (1.11–1.18) | 1.14 (1.10–1.18) | 1.15 (1.12–1.19) | 1.15 (1.11–1.19) | 1.18 (1.14–1.21) | 1.17 (1.13–1.21) |
| Shockable primary rhythm | 0.34 (0.28–0.40) | 0.31 (0.25–0.37) | 0.33 (0.28–0.39) | 0.32 (0.26–0.38) | 0.36 (0.31–0.41) | 0.35 (0.30–0.42) |
| Bystander witnessed OHCA | 0.69 (0.55–0.86) | 0.78 (0.61–0.997) | 0.68 (0.55–0.84) | 0.76 (0.60–0.96) | 0.69 (0.56–0.84) | 0.72 (0.58–0.90) |
| CCI of 3 or greater (cancer excluded) | 1.6 (1.32–1.95) | 1.32 (1.07–1.64) | 1.72 (1.43–2.08) | 1.42 (1.16–1.74) | 1.91 (1.60–2.27) | 1.59 (1.31–1.92) |
| Time to ROSC (pr. 5 minutes) | 1.08 (1.05–1.11) | 1.14 (1.11–1.18) | 1.07 (1.04–1.10) | 1.13 (1.10–1.17) | 1.05 (1.02–1.08) | 1.11 (1.07–1.14) |
CCI: Charlson comorbidity index; CI: confidence interval; HR: hazard ratio; OHCA: out-of-hospital cardiac arrest; ROSC: return of spontaneous circulation.
Factors associated with 30-day and 1-year mortality in patients with and without cancer prior to successful resuscitation from OHCA in univariate and multivariate analyses.
| . | 30-Day mortality . | 1-Year mortality . | End of follow-up (no cancer: mean 811 days; cancer: mean 406 days) . | |||
|---|---|---|---|---|---|---|
| . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . |
| Cancer prior to OHCA | 1.35 (1.07–1.71) | 0.98 (0.76–1.27) | 1.41 (1.13–1.76) | 0.99 (0.78–1.27) | 1.49 (1.21–1.83) | 1.01 (0.80–1.27) |
| Male sex | 0.64 (0.54–0.76) | 0.93 (0.77–1.13) | 0.63 (0.53–0.74) | 0.92 (0.77–1.11) | 0.64 (0.54–0.74) | 0.92 (0.77–1.10) |
| Age (5-year increase) | 1.14 (1.11–1.18) | 1.14 (1.10–1.18) | 1.15 (1.12–1.19) | 1.15 (1.11–1.19) | 1.18 (1.14–1.21) | 1.17 (1.13–1.21) |
| Shockable primary rhythm | 0.34 (0.28–0.40) | 0.31 (0.25–0.37) | 0.33 (0.28–0.39) | 0.32 (0.26–0.38) | 0.36 (0.31–0.41) | 0.35 (0.30–0.42) |
| Bystander witnessed OHCA | 0.69 (0.55–0.86) | 0.78 (0.61–0.997) | 0.68 (0.55–0.84) | 0.76 (0.60–0.96) | 0.69 (0.56–0.84) | 0.72 (0.58–0.90) |
| CCI of 3 or greater (cancer excluded) | 1.6 (1.32–1.95) | 1.32 (1.07–1.64) | 1.72 (1.43–2.08) | 1.42 (1.16–1.74) | 1.91 (1.60–2.27) | 1.59 (1.31–1.92) |
| Time to ROSC (pr. 5 minutes) | 1.08 (1.05–1.11) | 1.14 (1.11–1.18) | 1.07 (1.04–1.10) | 1.13 (1.10–1.17) | 1.05 (1.02–1.08) | 1.11 (1.07–1.14) |
| . | 30-Day mortality . | 1-Year mortality . | End of follow-up (no cancer: mean 811 days; cancer: mean 406 days) . | |||
|---|---|---|---|---|---|---|
| . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . | Univariate HR (95% CI) . | Multivariate HR (95% CI) . |
| Cancer prior to OHCA | 1.35 (1.07–1.71) | 0.98 (0.76–1.27) | 1.41 (1.13–1.76) | 0.99 (0.78–1.27) | 1.49 (1.21–1.83) | 1.01 (0.80–1.27) |
| Male sex | 0.64 (0.54–0.76) | 0.93 (0.77–1.13) | 0.63 (0.53–0.74) | 0.92 (0.77–1.11) | 0.64 (0.54–0.74) | 0.92 (0.77–1.10) |
| Age (5-year increase) | 1.14 (1.11–1.18) | 1.14 (1.10–1.18) | 1.15 (1.12–1.19) | 1.15 (1.11–1.19) | 1.18 (1.14–1.21) | 1.17 (1.13–1.21) |
| Shockable primary rhythm | 0.34 (0.28–0.40) | 0.31 (0.25–0.37) | 0.33 (0.28–0.39) | 0.32 (0.26–0.38) | 0.36 (0.31–0.41) | 0.35 (0.30–0.42) |
| Bystander witnessed OHCA | 0.69 (0.55–0.86) | 0.78 (0.61–0.997) | 0.68 (0.55–0.84) | 0.76 (0.60–0.96) | 0.69 (0.56–0.84) | 0.72 (0.58–0.90) |
| CCI of 3 or greater (cancer excluded) | 1.6 (1.32–1.95) | 1.32 (1.07–1.64) | 1.72 (1.43–2.08) | 1.42 (1.16–1.74) | 1.91 (1.60–2.27) | 1.59 (1.31–1.92) |
| Time to ROSC (pr. 5 minutes) | 1.08 (1.05–1.11) | 1.14 (1.11–1.18) | 1.07 (1.04–1.10) | 1.13 (1.10–1.17) | 1.05 (1.02–1.08) | 1.11 (1.07–1.14) |
CCI: Charlson comorbidity index; CI: confidence interval; HR: hazard ratio; OHCA: out-of-hospital cardiac arrest; ROSC: return of spontaneous circulation.
When excluding basal and squamous cell skin cancer, cancer was in univariate analysis associated with 30-day as well as 1-year mortality (HR30 days 1.44, 95% CI 1.13–1.83; HR1 year 1.47, 95% CI 1.17–1.86; Table 2, Figure 3); however, after adjustment for prognostic factors the difference was no longer present (HR30 days 1.05, 95% CI 0.80–1.38; HR1 year 1.05, 95% CI 0.81–1.36).
Neurological outcome
The number of patients discharged with a favourable neurological outcome was not found to differ between patients with and without cancer at hospital discharge (CPC 1/2 n=31 (26%) vs. n=310 (36%), P=0.08) or in a favourable outcome when assessing only surviving patients (CPC 5 excluded) (CPC 1 or 2 n=310 (84%) vs. n=31 (84%), P=1). In multiple logistic regression cancer was not associated with the odds of a favourable outcome (OR 1.16, 95% CI 0.47–3.32, P=0.77) when adjusting for prognostic factors.
Discussion
In this study we found that of 993 patients resuscitated from OHCA during the 4-year study period 12% of the cohort had a cancer diagnosis when assessing diagnoses back to 1994. Breast cancer, basal and squamous cell skin cancer, prostate cancer, pulmonary/pleural cancer and gastrointestinal cancer were the most common cancer types. Cancer was associated with the lower use of TTM in adjusted analysis, but no other differences in the use of post-resuscitation care were found. A favourable neurological outcome in surviving patients at discharge did not differ between patients with and without cancer. In unadjusted analysis, cancer was associated with higher 30-day, 1-year and mortality at end of follow-up, but after adjustment for prognostic factors; a difference was no longer present.
The most recent Danish cancer statistics showed a small decrease in incidence,22 while survival increases,23 which has increased the cancer prevalence significantly in recent years. This is in line with data from the USA10 and Europe,24 even though incidence and mortality vary across cancer types.10,22,–24 This highlights the need for information on prognosis after OHCA in cancer survivors, as the number of OHCA patients with a history of cancer is likely to increase.
Many studies on cardiac arrest and cancer have focused on resuscitation from IHCA.11,15,18 A recent study found no difference in survival between OHCA patients without cancer, OHCA patients with cancer and IHCA patients with cancer.25 Others again have found that ROSC rates did not change after IHCA in a cancer centre from 2002 to 2012 and survival to discharge was approximately 10%, which is similar to OHCA survival rates.26
It is likely that OHCA and IHCA patients with cancer differ, as the IHCA cohort may have a higher frequency of terminal patients and more acutely ill patients. On the other hand, terminally ill cancer patients may have do-not-resuscitate orders and thus may not be included in IHCA studies. Some studies of OHCA and cancer only include patients with active malignancies defined as requiring therapy.14 Patients in such studies may be more affected by their cancer than the OHCA patients in our cohort who had many different and not necessarily active cancers. To some extent, the inclusion of all prior cancer diagnoses in our study may explain the lack of difference in mortality between patients with and without cancer compared to other studies.13,14 We did not find differences between patients with active cancer compared to patients without cancer, but on the other hand we could not determine if therapy was needed in active cancers as no data on cancer therapy at the time of OHCA were available.
In the current study we found unadjusted differences in post-resuscitation care between patients with and without cancer, but when taking prognostic factors into account, only lower odds of TTM were found even though awake patients were excluded. We cannot determine whether this was due to a higher degree of frailty or comorbidities beyond what was captured by the CCI, or rather a chance finding. ICU admission, revascularisation, echocardiography, acute CAG, consultation with a cardiologist and the use of an ICD/pacemaker did not differ, indicating a sufficient work-up of OHCA patients, irrespective of cancer history. Others have found a lower use of post-resuscitation care of OHCA patients with and without active malignancy,14 as well as a lower use of guideline-recommended treatment in acute myocardial infarction patients with and without cancer.27 The reason for the discrepancy between these studies and ours may be that patients in the study by Kang et al. had active malignancies and very low 1-year survival, and some procedures may have been unethical or futile in very ill patients.14 As opposed to the study finding lower use of guideline-recommended therapies between acute myocardial infarction patients with and without cancer;27 we did not have information on the use of medication available. In this type of study it is not possible to infer whether cancer diagnosis affected triaging and level of care, but this cannot be ruled out. We found that patients with cancer more often had inhospital care terminated due to comorbidity, but we do not know if the comorbidity was the cancer diagnosis or another comorbidity.
This study deals with successfully resuscitated OHCA patients, and thus a probable difference in outcome of the resuscitation attempt, based on cancer prior to OHCA is not included. However in the prehospital acute setting during the resuscitation attempt the patient’s comorbidity and other prognostic factors may be quite difficult to access. Do-not-resuscitate orders may not be known in the acute setting. We do not know whether fewer patients with a history of cancer were successfully resuscitated. A qualitative study from Norway found that EMS personnel often find the resuscitation of cancer patients ethically challenging,28 this would probably apply in this study as well. It has previously been found that the most common cause for withholding or terminating resuscitation in our study area from June 2002 to May 2003 was prolonged anoxia.29 EMS personnel may not have access to information on cancer prior to OHCA, or may not be able to use the information in the acute situation in cases in which the cancer diagnosis is not recent. In general, there may be a risk of selection bias in this study.
Limitations
As this is a retrospective and observational study, it is not possible to detect causal relationships, but only associations. Residual confounding and selection bias cannot be ruled out. Furthermore, the relatively low number of patients with cancer may limit the power of subgroup analyses and increase the risk of overfitting on adjusting for confounders. To some extent, the cancer patients in this study may be considered a healthy cohort, as we cannot rule out that the proportion of cancer patients who could not be successfully resuscitated is high compared with those who could be resuscitated. Missingness was present in our data, especially in prehospital variables, but not in outcome.
Conclusion
In this study cancer diagnosis was associated with crude mortality after OHCA but no longer after adjustment for confounders, which was also true when assessing mortality in patients with active (<5 years prior to OHCA) and previous (⩾5 years prior to OHCA) cancer. Neurological outcoms did not differ between patients with and without cancer. Cancer patients had lower odds of TTM post-OHCA, but there were no other differences in post-resuscitation care between patients with and without cancer. According to the data of the current study, a cancer diagnosis should therefore be used with caution when prognostication in patients resuscitated from OHCA is performed.
Dr. Køber reports personal fees from Novartis, outside the submitted work. Dr. Kjaergaard reports grants from AstraZeneca, grants from Orion Pharma, grants from Bayer, outside the submitted work. Dr. Hassager reports personal fees from Novartis, personal fees from TEVA, personal fees from ViCare, outside the submitted work. Drs. Lippert and Søholm and MSc Winther-Jensen report no competing interests.
Dr Lippert reports unrestricted research grants from the Danish foundation TrygFonden and the Laerdal Foundation. M Winther-Jensen reports receiving grants from Rigshospitalets Forskningspuljer.




Comments