-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos L Alviar, Alejandra Gutierrez, Leslie Cho, Amar Krishnaswamy, Amr Saleh, Michael A Lincoff, Eric Roselli, Michael Militello, Venu Menon, Clevidipine as a therapeutic and cost-effective alternative to sodium nitroprusside in patients with acute aortic syndromes, European Heart Journal. Acute Cardiovascular Care, Volume 9, Issue 3_suppl, 1 October 2020, Pages S5–S12, https://doi.org/10.1177/2048872618777919
Close - Share Icon Share
Sodium nitroprusside is the preferred agent for the treatment of high blood pressure during acute aortic syndrome if blood pressure remains elevated after heart rate control with beta-blockers. The increasing cost of sodium nitroprusside in the USA led us to assess the efficacy and safety of intravenous clevidipine, a calcium channel blocker with quick onset of action, short half-life and significantly lower costs than sodium nitroprusside, in patients presenting with acute aortic syndrome.
We performed a retrospective chart review of consecutive patients admitted to the Cleveland Clinic Cardiac Intensive Care Unit from 2013–2016 with a diagnosis of acute aortic syndrome. Patients who received intravenous sodium nitroprusside were compared with those receiving intravenous clevidipine. The primary outcome was a significant difference in blood pressure at one, three and six hours. Secondary outcomes included time to achieving blood pressure target and in hospital mortality with rates of hypotension and bradycardia as safety endpoints.
A total of 85 patients with suspected acute aortic pathology received clevidipine and 50 received sodium nitroprusside. Clinical and demographic characteristics were similar in both groups, except for a higher incidence of abdominal aortic aneurysm in the clevidipine group and for a trend towards higher use of labetalol in the clevidipine group. There were no significant differences in blood pressure or heart rate at one, three and six hours after starting either infusion. The rates of hypotension, bradycardia and in-hospital mortality did not differ. Time to achieve blood pressure control were also similar between groups.
Intravenous clevidipine appears to be a safe and effective alternative to sodium nitroprusside for the management of high blood pressure during acute aortic dissection. In the USA, clevidipine could represent a cost effective therapy providing similar outcomes than sodium nitroprusside.
Introduction
The main objective of treatment of patients with acute aortic syndrome (AAS) including aortic dissection, intramural hematoma, and penetrating aortic ulcer is to decrease shear stress in the aortic wall1 while proceeding to definitive surgery if indicated. This therapeutic goal relies on prompt and effective blood pressure (BP) control, usually performed in critical care settings, with a target systolic blood pressure (SBP) of <120 mm Hg that is aimed after a target heart rate of <60 beats per minute (bpm) has been achieved.2 This approach has the purpose of decreasing dissection progression or hematoma expansion,1,–3 and it is usually achieved by combining intravenous beta-blockers along with potent and rapid-acting systemic vasodilators that are started immediately after patient presentation.4,5 Intravenous sodium nitroprusside (SNP), due to its rapid onset, short half-life, and convenience in drug titration,6 has been classically utilized for the treatment of acute hypertension in the setting of aortic dissection, primarily based on data published in patients with hypertensive crisis or perioperative hypertension.7,,–10 SNP leads to both venous and arterial dilatation, by promoting nitric oxide (NO) release and by stimulating guanylyl cyclase to produce cyclic guanosine monophosphate (GMP) in order to sequester intracellular calcium and inhibit cellular contraction.11,,–14 Moreover many physicians choose this agent due to their familiarity with it from long standing use of it in clinical practice.
Recent changes in the manufacturing of SNP, have resulted in an exponential increase in the cost of this drug over the last 2–3 years. This near prohibitive increase in pricing has markedly escalated pharmacy costs in the cardiac intensive care unit (CICU) and raised significant concerns amongst clinicians and hospital administrators.15,16 Currently, there is a paucity of studies comparing the effectiveness and safety of the available intravenous agents for the management of acute hypertension in patients with AAS.8,17 Faced with this economic challenge, we evaluated the efficacy of clevidipine for BP control in this setting.
Clevidipine is an intravenous dihydropyridine calcium channel blocker (CCB) that was first approved in the USA in 2008 for the management of acute hypertension.18 The chemical structure of clevidipine is closely related to femlodipine, but its actions and pharmacodynamic effects are more similar to nicardipine, an agent that has been used since the 1990s.19 The main advantage of clevidipine over nicardipine is its faster onset of action, which is about 2–4 min compared to 5–10 min for nicardipine, and equally important is its ultra-short half-life, which is 5–15 min compared to 4–6 h for nicardipine.20,21 This characteristic enables rapid recovery of BP in cases when the antihypertensive effect is pronounced leading to an overshoot phenomenon and symptomatic hypotension. Clevidipine has been mostly used in hypertensive crises or for BP control in the perioperative period.18 However, it is a potential useful alternative in patients with aortic dissection who required further BP control after proper heart rate control with beta blockade. The lower cost of clevidipine when compared to SNP, as well as its potent and short onset and offset of action makes it a very attractive agent for the practice of cost-effective medical care. Therefore, our aim is to report our experience of treating hypertension with this agent in patients presenting with AAS and compare it with SNP for the same indication.
Methods
Our study population consisted of consecutive patients admitted to the Cleveland Clinic coronary intensive care unit from 2013–2016 who received either sodium nitroprusside or clevidipine per pharmacy dispensing records. Patients who were admitted for AAS were identified by careful chart review of the electronic medical record. Patients without a confirmed diagnosis of AAS and those who had incomplete hemodynamic data were excluded from the study. Similarly, patients who received both clevidipine and SNP within the first 24-hour period were also excluded.
Data collection was performed by retrospective review of electronic medical records by two independent reviewers. This included abstraction of data from inpatient notes, medication records and flow chart. According to the standard Cleveland Clinic protocol for the management of AAS, all patients admitted to the coronary care unit with a diagnosis of AAS had continuous intra-arterial monitoring via the radial artery and hemodynamic data was frequently documented enabling registry and correlation between the vital signs and the dosage and types of medications administered. Similarly, all patients had an echocardiogram performed at the time of admission to the unit. Primary study endpoints included hemodynamic parameters such as BP and heart rate on admission at one, three, and six hours, with the main primary endpoint being SBP at one hour. Secondary endpoints included time taken to achieve BP target (SBP ⩽120 mm Hg) and in hospital death. Rates of hypotension defined as SBP<100 mm Hg and bradycardia <50 bpm were ascertained as safety endpoints. All the hemodynamic data was obtained from the electronic medical records as stated above. The validity of the hemodynamic data obtained from the electronic medical records is based on the fact that every data point that was input into the electronic system was validated and entered manually by the bedside nurse certified in critical care nursing. Other data collected included demographic characteristics, comorbid conditions, additional medications used and final diagnosis.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 22 (IBM Corporation, Armonk, New York, USA). Descriptive statistics were used to characterize the population in each of the collected variables. The clinical and demographic variables were compared using parametric or non-parametric testing according to distribution of the data. For continuous variables one-way analysis of variance (ANOVA) was used for normally distributed data or the Kruskal-Wallis test was used for non-normally distributed data. Chi square or Fisher’s exact test analysis was used to compare nominal variables between groups depending on data distribution. Assessment for normality was performed using the Kolmogorov-Smirnoff test. Data was presented as mean and standard deviation and percentages as appropriate. A p of 0.05 was used for significance.
Results
Clinical characteristics
A total of 330 patients were initially screened, based on pharmacy dispensing records as receiving SNP or clevidipine. From these, a total of 135 patients were identified from pharmacy records as having the diagnosis of AAS and the remaining patients were excluded based on the exclusion criteria. The final sample consisted of 85 subjects treated who received clevidipine and 50 who received SNP (Figure 1). Baseline characteristics are shown in Table 1. There were no significant differences in comorbid conditions between patients receiving clevidipine and those treated with SNP. Half of the patients were male (56%) and the mean age was of 64±16 years. The majority of the patients had hypertension and more than a third of them had established coronary artery disease and history of smoking.
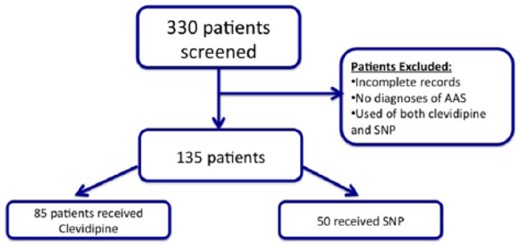
Consort diagram on patient selection. AAS: acute aortic syndrome; SNP: sodium nitroprusside.
Baseline characteristics of patients with acute aortic syndrome (AAS) treated with clevidipine compared to sodium nitroprusside (SNP).
| . | Clevidipine (n=85) . | SNP (n=50) . | p Value . |
|---|---|---|---|
| Clinical characteristics | |||
| Gender, male – n (%) | 48 (56.0%) | 27 (54.0%) | 0.858 |
| Age, years, mean ± SD | 65.4±15.4 | 61.3±16.4 | 0.151 |
| Coronary artery disease – n (%) | 15 (30.0%) | 27 (31.8%) | 0.850 |
| Hypertension – n (%) | 73 (85.9%) | 46 (92.0%) | 0.410 |
| HfrEF – n (%) | 3 (3.5%) | 6 (12.0%) | 0.076 |
| HfpEF – n (%) | 3 (3.5%) | 0 (0.0%) | NA |
| Atrial fibrillation – n (%) | 20 (23.5%) | 8 (16.0%) | 0.381 |
| Hyperlipidemia – n (%) | 36 (42.4%) | 18 (36.0%) | 0.586 |
| Smoking – n (%) | 27 (31.8%) | 18 (36.0%) | 0.706 |
| Diabetes mellitus – n (%) | 11 (12.9%) | 5 (10.0%) | 0.784 |
| CKD – n (%) | 11 (12.9%) | 4 (8.0%) | 0.572 |
| Dialysis – n (%) | 0 (0.0%) | 1 (2.0%) | 0.370 |
| Ejection fraction, mean±SD | 60.05±7.7 | 57.5±11.3 | 0.170 |
| Serum creatinine, mean±SD | 1.45±3.6 | 1.1±0.5 | 0.380 |
| SBP at presentation, mean±SD | 146.3±25.8 | 155.5±29.9 | 0.080 |
| DBP at presentation, mean±SD | 74.6±16.3 | 74.62±18.9 | 0.900 |
| HR at presentation, mean±SD | 74.01±13.8 | 73.14±12.4 | 0.700 |
| Type of aortic pathology | |||
| Type A AAD – n (%) | 27 (31.8%) | 23 (46.0%) | 0.139 |
| Type B AAD – n (%) | 44 (51.8%) | 33 (66.0%) | 0.149 |
| AAA – n (%) | 30 (35.7%) | 7 (14.0%) | 0.009 |
| Concomitant medications | |||
| Metoprolol – n (%) | 73 (85.9%) | 42 (84.0%) | 0.805 |
| Labetalol – n (%) | 14 (16.7%) | 16 (32.0%) | 0.053 |
| Diltiazem – n (%) | 5 (5.9%) | 1 (2.0%) | 0.411 |
| . | Clevidipine (n=85) . | SNP (n=50) . | p Value . |
|---|---|---|---|
| Clinical characteristics | |||
| Gender, male – n (%) | 48 (56.0%) | 27 (54.0%) | 0.858 |
| Age, years, mean ± SD | 65.4±15.4 | 61.3±16.4 | 0.151 |
| Coronary artery disease – n (%) | 15 (30.0%) | 27 (31.8%) | 0.850 |
| Hypertension – n (%) | 73 (85.9%) | 46 (92.0%) | 0.410 |
| HfrEF – n (%) | 3 (3.5%) | 6 (12.0%) | 0.076 |
| HfpEF – n (%) | 3 (3.5%) | 0 (0.0%) | NA |
| Atrial fibrillation – n (%) | 20 (23.5%) | 8 (16.0%) | 0.381 |
| Hyperlipidemia – n (%) | 36 (42.4%) | 18 (36.0%) | 0.586 |
| Smoking – n (%) | 27 (31.8%) | 18 (36.0%) | 0.706 |
| Diabetes mellitus – n (%) | 11 (12.9%) | 5 (10.0%) | 0.784 |
| CKD – n (%) | 11 (12.9%) | 4 (8.0%) | 0.572 |
| Dialysis – n (%) | 0 (0.0%) | 1 (2.0%) | 0.370 |
| Ejection fraction, mean±SD | 60.05±7.7 | 57.5±11.3 | 0.170 |
| Serum creatinine, mean±SD | 1.45±3.6 | 1.1±0.5 | 0.380 |
| SBP at presentation, mean±SD | 146.3±25.8 | 155.5±29.9 | 0.080 |
| DBP at presentation, mean±SD | 74.6±16.3 | 74.62±18.9 | 0.900 |
| HR at presentation, mean±SD | 74.01±13.8 | 73.14±12.4 | 0.700 |
| Type of aortic pathology | |||
| Type A AAD – n (%) | 27 (31.8%) | 23 (46.0%) | 0.139 |
| Type B AAD – n (%) | 44 (51.8%) | 33 (66.0%) | 0.149 |
| AAA – n (%) | 30 (35.7%) | 7 (14.0%) | 0.009 |
| Concomitant medications | |||
| Metoprolol – n (%) | 73 (85.9%) | 42 (84.0%) | 0.805 |
| Labetalol – n (%) | 14 (16.7%) | 16 (32.0%) | 0.053 |
| Diltiazem – n (%) | 5 (5.9%) | 1 (2.0%) | 0.411 |
AAA: abdominal aortic aneurysm; AAD: acute aortic dissection; CKD: chronic kidney disease; DBP: diastolic blood pressure HfpEF: heart failure with preserved ejection fraction; HfrEF: heart failure with reduced ejection fraction; HR: heart rate; SBP: systolic blood pressure; SD: standard deviation.
Baseline characteristics of patients with acute aortic syndrome (AAS) treated with clevidipine compared to sodium nitroprusside (SNP).
| . | Clevidipine (n=85) . | SNP (n=50) . | p Value . |
|---|---|---|---|
| Clinical characteristics | |||
| Gender, male – n (%) | 48 (56.0%) | 27 (54.0%) | 0.858 |
| Age, years, mean ± SD | 65.4±15.4 | 61.3±16.4 | 0.151 |
| Coronary artery disease – n (%) | 15 (30.0%) | 27 (31.8%) | 0.850 |
| Hypertension – n (%) | 73 (85.9%) | 46 (92.0%) | 0.410 |
| HfrEF – n (%) | 3 (3.5%) | 6 (12.0%) | 0.076 |
| HfpEF – n (%) | 3 (3.5%) | 0 (0.0%) | NA |
| Atrial fibrillation – n (%) | 20 (23.5%) | 8 (16.0%) | 0.381 |
| Hyperlipidemia – n (%) | 36 (42.4%) | 18 (36.0%) | 0.586 |
| Smoking – n (%) | 27 (31.8%) | 18 (36.0%) | 0.706 |
| Diabetes mellitus – n (%) | 11 (12.9%) | 5 (10.0%) | 0.784 |
| CKD – n (%) | 11 (12.9%) | 4 (8.0%) | 0.572 |
| Dialysis – n (%) | 0 (0.0%) | 1 (2.0%) | 0.370 |
| Ejection fraction, mean±SD | 60.05±7.7 | 57.5±11.3 | 0.170 |
| Serum creatinine, mean±SD | 1.45±3.6 | 1.1±0.5 | 0.380 |
| SBP at presentation, mean±SD | 146.3±25.8 | 155.5±29.9 | 0.080 |
| DBP at presentation, mean±SD | 74.6±16.3 | 74.62±18.9 | 0.900 |
| HR at presentation, mean±SD | 74.01±13.8 | 73.14±12.4 | 0.700 |
| Type of aortic pathology | |||
| Type A AAD – n (%) | 27 (31.8%) | 23 (46.0%) | 0.139 |
| Type B AAD – n (%) | 44 (51.8%) | 33 (66.0%) | 0.149 |
| AAA – n (%) | 30 (35.7%) | 7 (14.0%) | 0.009 |
| Concomitant medications | |||
| Metoprolol – n (%) | 73 (85.9%) | 42 (84.0%) | 0.805 |
| Labetalol – n (%) | 14 (16.7%) | 16 (32.0%) | 0.053 |
| Diltiazem – n (%) | 5 (5.9%) | 1 (2.0%) | 0.411 |
| . | Clevidipine (n=85) . | SNP (n=50) . | p Value . |
|---|---|---|---|
| Clinical characteristics | |||
| Gender, male – n (%) | 48 (56.0%) | 27 (54.0%) | 0.858 |
| Age, years, mean ± SD | 65.4±15.4 | 61.3±16.4 | 0.151 |
| Coronary artery disease – n (%) | 15 (30.0%) | 27 (31.8%) | 0.850 |
| Hypertension – n (%) | 73 (85.9%) | 46 (92.0%) | 0.410 |
| HfrEF – n (%) | 3 (3.5%) | 6 (12.0%) | 0.076 |
| HfpEF – n (%) | 3 (3.5%) | 0 (0.0%) | NA |
| Atrial fibrillation – n (%) | 20 (23.5%) | 8 (16.0%) | 0.381 |
| Hyperlipidemia – n (%) | 36 (42.4%) | 18 (36.0%) | 0.586 |
| Smoking – n (%) | 27 (31.8%) | 18 (36.0%) | 0.706 |
| Diabetes mellitus – n (%) | 11 (12.9%) | 5 (10.0%) | 0.784 |
| CKD – n (%) | 11 (12.9%) | 4 (8.0%) | 0.572 |
| Dialysis – n (%) | 0 (0.0%) | 1 (2.0%) | 0.370 |
| Ejection fraction, mean±SD | 60.05±7.7 | 57.5±11.3 | 0.170 |
| Serum creatinine, mean±SD | 1.45±3.6 | 1.1±0.5 | 0.380 |
| SBP at presentation, mean±SD | 146.3±25.8 | 155.5±29.9 | 0.080 |
| DBP at presentation, mean±SD | 74.6±16.3 | 74.62±18.9 | 0.900 |
| HR at presentation, mean±SD | 74.01±13.8 | 73.14±12.4 | 0.700 |
| Type of aortic pathology | |||
| Type A AAD – n (%) | 27 (31.8%) | 23 (46.0%) | 0.139 |
| Type B AAD – n (%) | 44 (51.8%) | 33 (66.0%) | 0.149 |
| AAA – n (%) | 30 (35.7%) | 7 (14.0%) | 0.009 |
| Concomitant medications | |||
| Metoprolol – n (%) | 73 (85.9%) | 42 (84.0%) | 0.805 |
| Labetalol – n (%) | 14 (16.7%) | 16 (32.0%) | 0.053 |
| Diltiazem – n (%) | 5 (5.9%) | 1 (2.0%) | 0.411 |
AAA: abdominal aortic aneurysm; AAD: acute aortic dissection; CKD: chronic kidney disease; DBP: diastolic blood pressure HfpEF: heart failure with preserved ejection fraction; HfrEF: heart failure with reduced ejection fraction; HR: heart rate; SBP: systolic blood pressure; SD: standard deviation.
The baseline BP on CICU arrival did not differ between both groups with a mean SBP of 138 mm Hg and a mean DBP of 70 mm Hg in the clevidipine group and mean SBP of 140 mm Hg and a mean DBP of 73 mm Hg in the SNP group (p=0.08 and 0.90 respectively). The predominant aortic pathology was acute type B aortic dissection in both groups. There were fewer patients with an abdominal aortic aneurysm in the SNP group than in the clevidipine group (14 vs 36%; p=0.009). An echocardiogram performed on admission demonstrated a mean ejection fraction of 59±10% for the entire cohort, and it was no different between patients receiving SNP or clevidipine. A Left ventricular ejection fraction (LVEF)<45% was noticed in 7% of the population regardless of treatment. As expected, most patients had concomitant treatment with a beta-blocker, and the agent most commonly used was metoprolol. Patients who were treated with SNP were treated with additional labetalol more often than those treated with clevidipine (32 vs 17%; p=0.05).
BP control and heart rate response between clevidipine and SNP
As shown in Figure 2(a), there were no differences in achieving adequate BP control with either agent. There were no significant differences in average SBP and DBP between groups at one, three, and six hours after starting the infusion of either agent. Of note, at three hours after infusion, patients treated with clevidipine had a slightly lower BP than those treated with SNP (119±16 vs 125±20 mm Hg), however this difference was not statistically significant and it was only represented by a trend (p=0.05). Heart rate was not significantly different at baseline, one and three hours after starting the infusion of either agent. However, at six hours, patients who received clevidipine had a lower heart rate than those who received SNP (68.8±0.4 vs 75.6±12.5 bpm; p=0.001), as seen in Figure 2(b).
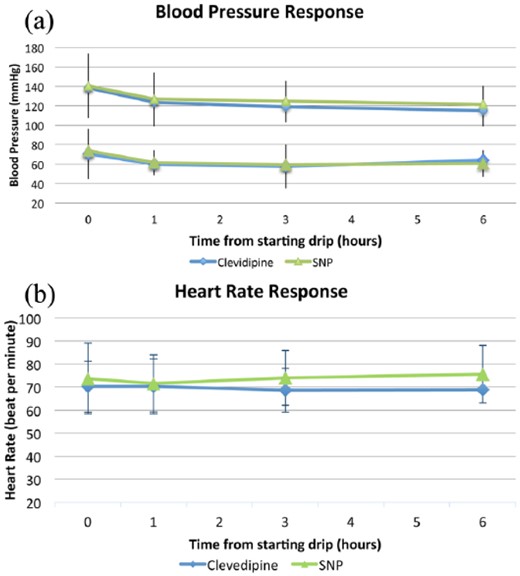
Change in hemodynamic parameters in patients on clevidipine and sodium nitroprusside (SNP). (a) Systolic and diastolic blood pressure at baseline, one, three, and six hours after starting the infusion. (b) Heart rate at baseline, one, three, and six hours after starting the infusion.
Secondary endpoints between clevidipine and SNP infusion
Clinical outcomes are shown in Table 2. The time to reach average target SBP (120 mm Hg) was not statistically significant (2.18±2.7 h for clevidipine vs 2.21±2.19 for SNP, p=0.9) Regarding safety, clevidipine use was associated with a higher incidence of bradycardia when compared to SNP (26% vs 8%; p=0.012). Notably, while the use of labetalol was higher in the SNP group, metoprolol use was no different between groups, however the cumulative dose and frequency of administration of these medications was not recorded. There was no significant difference in in-hospital mortality or in the incidence of hypotension between both groups.
Outcomes of patients with acute aortic syndrome (AAS) who received clevidipine compared to sodium nitroprusside (SNP).
| . | Clevidipine n=85 . | SNP n=50 . | p Value . |
|---|---|---|---|
| SBP when starting infusion, mean±SD | 138.4±27.7 | 140.5±33.1 | 0.710 |
| DBP when starting infusion, mean±SD | 70.5±25.8 | 68.1±17.6 | 0.523 |
| SBP at 1 h, mean±SD | 123.6±18 | 126.8±27.6 | 0.500 |
| DBP at 1 h, mean±SD | 60.1±11.7 | 61.6±12.7 | 0.500 |
| SBP at 3 h, mean±SD | 119.1±16 | 125.0±20.3 | 0.050 |
| DBP at 3 h, mean±SD | 57.6±22.4 | 59.4±10.3 | 0.340 |
| SBP at 6 h, mean±SD | 115.05±16.0 | 121.3±19.3 | 0.060 |
| DBP at 6 h, mean±SD | 64.3±9.0 | 60.8±13.6 | 0.600 |
| HR When starting infusion, mean±SD | 70.2±11.1 | 73.7±15.4 | 0.170 |
| HR at 1 h, mean±SD | 70.25±12.0 | 71.56±12.31 | 0.500 |
| HR at 3 h, mean±SD | 68.6±9.5 | 71.5±11.9 | 0.170 |
| HR at 6 h, mean±SD | 68.8±9.4 | 75.6±12.5 | 0.001 |
| Time to achieve target BP (h) | 2.18±2.7 | 2.21±2.19 | 0.900 |
| Adverse events | |||
| Hypotension – n (%) | 40 (47.6%) | 16 (32.0%) | 0.103 |
| Bradycardia – n (%) | 22 (26.5%) | 4 (8.0%) | 0.012 |
| In-hospital mortality – n (%) | 9 (10.6%) | 7 (14.0%) | 0.588 |
| Length of stay (days), mean±SD | 8±7 | 10±9 | 0.208 |
| . | Clevidipine n=85 . | SNP n=50 . | p Value . |
|---|---|---|---|
| SBP when starting infusion, mean±SD | 138.4±27.7 | 140.5±33.1 | 0.710 |
| DBP when starting infusion, mean±SD | 70.5±25.8 | 68.1±17.6 | 0.523 |
| SBP at 1 h, mean±SD | 123.6±18 | 126.8±27.6 | 0.500 |
| DBP at 1 h, mean±SD | 60.1±11.7 | 61.6±12.7 | 0.500 |
| SBP at 3 h, mean±SD | 119.1±16 | 125.0±20.3 | 0.050 |
| DBP at 3 h, mean±SD | 57.6±22.4 | 59.4±10.3 | 0.340 |
| SBP at 6 h, mean±SD | 115.05±16.0 | 121.3±19.3 | 0.060 |
| DBP at 6 h, mean±SD | 64.3±9.0 | 60.8±13.6 | 0.600 |
| HR When starting infusion, mean±SD | 70.2±11.1 | 73.7±15.4 | 0.170 |
| HR at 1 h, mean±SD | 70.25±12.0 | 71.56±12.31 | 0.500 |
| HR at 3 h, mean±SD | 68.6±9.5 | 71.5±11.9 | 0.170 |
| HR at 6 h, mean±SD | 68.8±9.4 | 75.6±12.5 | 0.001 |
| Time to achieve target BP (h) | 2.18±2.7 | 2.21±2.19 | 0.900 |
| Adverse events | |||
| Hypotension – n (%) | 40 (47.6%) | 16 (32.0%) | 0.103 |
| Bradycardia – n (%) | 22 (26.5%) | 4 (8.0%) | 0.012 |
| In-hospital mortality – n (%) | 9 (10.6%) | 7 (14.0%) | 0.588 |
| Length of stay (days), mean±SD | 8±7 | 10±9 | 0.208 |
DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure; SD: standard deviation.
Outcomes of patients with acute aortic syndrome (AAS) who received clevidipine compared to sodium nitroprusside (SNP).
| . | Clevidipine n=85 . | SNP n=50 . | p Value . |
|---|---|---|---|
| SBP when starting infusion, mean±SD | 138.4±27.7 | 140.5±33.1 | 0.710 |
| DBP when starting infusion, mean±SD | 70.5±25.8 | 68.1±17.6 | 0.523 |
| SBP at 1 h, mean±SD | 123.6±18 | 126.8±27.6 | 0.500 |
| DBP at 1 h, mean±SD | 60.1±11.7 | 61.6±12.7 | 0.500 |
| SBP at 3 h, mean±SD | 119.1±16 | 125.0±20.3 | 0.050 |
| DBP at 3 h, mean±SD | 57.6±22.4 | 59.4±10.3 | 0.340 |
| SBP at 6 h, mean±SD | 115.05±16.0 | 121.3±19.3 | 0.060 |
| DBP at 6 h, mean±SD | 64.3±9.0 | 60.8±13.6 | 0.600 |
| HR When starting infusion, mean±SD | 70.2±11.1 | 73.7±15.4 | 0.170 |
| HR at 1 h, mean±SD | 70.25±12.0 | 71.56±12.31 | 0.500 |
| HR at 3 h, mean±SD | 68.6±9.5 | 71.5±11.9 | 0.170 |
| HR at 6 h, mean±SD | 68.8±9.4 | 75.6±12.5 | 0.001 |
| Time to achieve target BP (h) | 2.18±2.7 | 2.21±2.19 | 0.900 |
| Adverse events | |||
| Hypotension – n (%) | 40 (47.6%) | 16 (32.0%) | 0.103 |
| Bradycardia – n (%) | 22 (26.5%) | 4 (8.0%) | 0.012 |
| In-hospital mortality – n (%) | 9 (10.6%) | 7 (14.0%) | 0.588 |
| Length of stay (days), mean±SD | 8±7 | 10±9 | 0.208 |
| . | Clevidipine n=85 . | SNP n=50 . | p Value . |
|---|---|---|---|
| SBP when starting infusion, mean±SD | 138.4±27.7 | 140.5±33.1 | 0.710 |
| DBP when starting infusion, mean±SD | 70.5±25.8 | 68.1±17.6 | 0.523 |
| SBP at 1 h, mean±SD | 123.6±18 | 126.8±27.6 | 0.500 |
| DBP at 1 h, mean±SD | 60.1±11.7 | 61.6±12.7 | 0.500 |
| SBP at 3 h, mean±SD | 119.1±16 | 125.0±20.3 | 0.050 |
| DBP at 3 h, mean±SD | 57.6±22.4 | 59.4±10.3 | 0.340 |
| SBP at 6 h, mean±SD | 115.05±16.0 | 121.3±19.3 | 0.060 |
| DBP at 6 h, mean±SD | 64.3±9.0 | 60.8±13.6 | 0.600 |
| HR When starting infusion, mean±SD | 70.2±11.1 | 73.7±15.4 | 0.170 |
| HR at 1 h, mean±SD | 70.25±12.0 | 71.56±12.31 | 0.500 |
| HR at 3 h, mean±SD | 68.6±9.5 | 71.5±11.9 | 0.170 |
| HR at 6 h, mean±SD | 68.8±9.4 | 75.6±12.5 | 0.001 |
| Time to achieve target BP (h) | 2.18±2.7 | 2.21±2.19 | 0.900 |
| Adverse events | |||
| Hypotension – n (%) | 40 (47.6%) | 16 (32.0%) | 0.103 |
| Bradycardia – n (%) | 22 (26.5%) | 4 (8.0%) | 0.012 |
| In-hospital mortality – n (%) | 9 (10.6%) | 7 (14.0%) | 0.588 |
| Length of stay (days), mean±SD | 8±7 | 10±9 | 0.208 |
DBP: diastolic blood pressure; HR: heart rate; SBP: systolic blood pressure; SD: standard deviation.
Discussion
Our study compares the acute hemodynamic parameters, including BP control, time to achieve BP target, heart rate, hypotension rates, and mortality rates of two antihypertensive regimens, SNP and clevidipine, used in acute settings for the treatment of AAS.1 To our knowledge, this is the first study assessing alternative agents for BP control during AAS. Our data demonstrate that a strategy utilizing titratable intravenous clevidipine was as efficacious as utilizing SNP for the treatment of hypertension in the acute management of AAS. In a background of adjunctive beta-blockade, both clevidipine and SNP had similar BP control and mortality. We observed slightly higher rates of hypotension with the strategy utilizing clevidipine with a minimal decrease in heart rate that was not associated with adverse clinical consequences. The design of this study does not enable us to deduce whether this was intrinsic to the agent or the consequence of excess beta-blockade exposure in this group. Our findings are more likely related to adjunctive therapy as existing literature has shown no difference17 or a slight increase in heart rate associated with clevidipine use,8,14,–16,20 which is thought to be due to reflex tachycardia from arteriolar vasodilatation. The negative chronotropic effect of dihydropyridines is known to be negligible, even when combined with agents known to slow heart rate and conduction through the AC node, such as beta-blockers or non-dihydropyridine CCBs22 which is usually done for the treatment of AAS.
Interestingly, although the number of patients in our cohort with low ejection fraction was small, the similar hemodynamic response in these patients when compared to patients with normal ejection fraction, suggests that clevidipine might be safely tolerated in patients with systolic dysfunction, and supports prior data on the use of intravenous nicardipine in patients with low ejection fraction.23 However this should be further analyzed in prospective studies with larger cohorts, before making any definitive recommendations.
SNP has been traditionally used by many clinicians as the agent of choice to treat AAS, given its rapid onset of action, potency, short half-life, and its long record in cardiovascular medicine and anesthesia,6 as well as its initial low cost.12 However, in 2015 there were significant changes in the manufacturing and ownership of the rights of SNP, leading to a substantial rise in the price of the medication of more than 1500% in the last three years.15,16 This dramatic increase in the cost of SNP led clinicians and institutions to look for alternative options to treat acute hypertension (Figure 3). Among these alternative agents, clevidipine was considered as an appropriate choice.24 In the setting of cardiac surgery Cruz and colleagues have reported on successfully transitioning from SNP to clevidipine at their institution. Similarly, members of our group have recently published a strategy adopted in our institution with the aim of decreasing the cost of SNP, demonstrating how this approach could translate into significant projected savings of US$8,067,551.25 In the present study, the estimated average per diem cost of SNP in patients with acute aortic syndromes was US$1163 dollars, while the per diem cost of clevidipine for the same purpose was US$311 dollars.
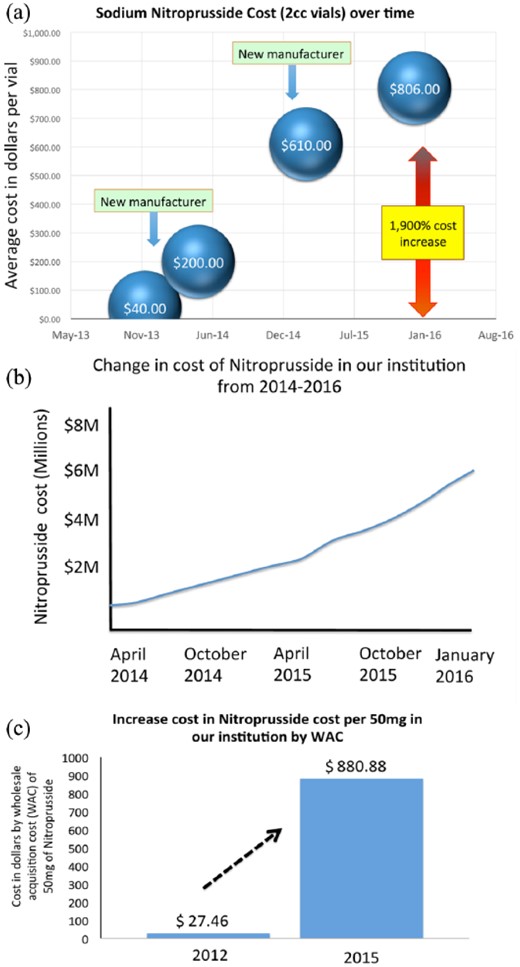
Change in cost of sodium nitroprusside over the last three years (US$). (a) Change in cost in the USA. (b) Change in total cost of sodium nitroprusside in our institution from 2014–2016. (c) Change in price paid by our institution to the manufacturer of sodium nitroprusside by wholesale acquisition cost.
Clevidipine is a dihydropyridine CCB that has a number of properties that make it useful agent in the setting of a suspected acute aortic dissection (AAD). It reaches a steady state concentration in 2–10 min and is rapidly metabolized by hydrolysis by blood esterases with a terminal elimination half-life of about 15 min.20,26 This fast onset and short half-life is highly desirable in the setting of AAD and has already proven effective for surgical and preoperative control of acute hypertension.21,26 It is a voltage dependent L- type CCB which due to the higher resting potential of vascular smooth muscle cells (compared to myocytes) is selective to the vascular smooth muscle cells and has a stronger effect in arteries than veins.27 This predominant arterial vasodilatation, prevents venous pooling and decreases hypotension.21 The high drug clearance with a small volume of distribution contributes to a very short half-life of 2–4 min making it an ideal agent for titration; a property that is vital in AAD with its potential for acute hemodynamic instability. This features also enables rapid recovery of BP in cases when the antihypertensive effect is pronounced leading to an overshoot phenomenon and hypotension.14 Finally its current cost in the USA is significantly cheaper than SNP.28 This is an important factor in the current era, as finding alternative agents to replace SNP in different clinical scenarios is a crucial need for many institutions. This is supported by recent data obtained from 47 hospitals in the USA that demonstrated a significant decrease in the use of SNP, with an observed drop of 56% patients receiving this medication from 2012–2015.29
Our study highlights that these benefits of clevidipine can be effectively utilized in the management of patients with AAS. Despite the use of two titratable agents, transient overshoot was common in our study, highlighting a challenge encountered by the clinicians at the bedside. The clinical utility of clevidipine adds to a number of studies in the literature supporting its use in acute hypertension, including patients undergoing CABG as well as in patients with hypertensive emergencies, in both placebo-controlled and active-comparison studies.21,27,30,,,,–35
Further reassurance for the use of clevidipine as a substitute for nitroprusside in the AAS setting is provided by the Evaluation of Clevidipine In the Perioperative Treatment of Hypertension Assessing Safety Events (ECLIPSE) trial.32 This was a prospective randomized open-label study that compared the safety and efficacy of clevidipine, nitroglycerin, SNP, and nicardipine in 1512 patients undergoing coronary artery bypass grafting. In this study, clevidipine was more effective than SNP in maintaining patients in their pre-specified BP range. In this study there were no differences in complication rates, including myocardial infarction, stroke, or renal dysfunction between patients treated with clevidipine and patients treated with other agents.
Given the relative safety and efficacy of clevidipine, as well as the significantly lower cost of this agent compared to SNP, it provides us with an excellent alternative for the management of AAS in the USA. While other countries still have access to SNP at a low cost,36 in the USA, SNP has almost became a “luxury medication,” with outrageous prices forcing clinicians to abandon or significantly restrict its use. In fact, at high volume centers like our institution, where the number of patients with acute aortic pathology goes beyond 210 cases per year, it is possible to significantly reduce healthcare costs by adopting the use of clevidipine instead of SNP.
Limitations
Although our study has limitations, it also has a number of strengths. While the retrospective observational nature of the study designs may render it vulnerable to bias from unmeasured confounders, the data presented here represents the largest experience with the use of clevidipine in AAS ever reported. Moreover, despite the non-randomized nature of our study the baseline characteristics between the two groups were fairly similar as seen in Table 1, except for a higher rate of abdominal aortic aneurysm (AAA) in the clevidipine group. This finding however, should not impact on our observations as it is unlikely to influence BP control targets in the intensive care unit. Given our inability to quantify the dosage of adjunctive beta-blockade in the two groups, we are unable to opine on dose and BP effect in this setting. The study however clearly confirms that a strategy of utilizing clevidipine in experienced hands is as effective as nitroprusside for the management of AAS patients treated with adjunctive beta blockade. The use of continuous arterial monitoring of BP and the documentation of BP in the electronic medical records (EMR) enables us to accurately ascertain the BP response in patients treated regardless of exposed therapy. The small sample limits our ability to properly analyze hard endpoints, such as mortality; however, that was not the focus of this observation. Similarly, we acknowledge the limitation of this being an observational study and therefore we did not perform a sample size calculation a priori. However, if we were to design a prospective, randomized, clinical trial comparing both treatments using SBP as the primary endpoint, the sample size needed would include a total of 44 patients (22 in each group). This is based on 0.8 statistical power, with an assumed a Type-I error rate of 0.05, a standard deviation of 4 mm Hg, a clevidipine group mean of 108 mm Hg, a SNP group mean of 106 mm Hg, and a non-inferiority margin of 5 mm Hg. This estimated sample size follows below the number of the patients included in this study, suggesting that if this were a prospective randomized trial, it would be sufficiently powered to assess for non-inferiority of clevidipine in regards of SBP. In terms of data collection, we did not assess the frequency and dosages of the two medications and could therefore not control for the effect of cumulative doses of either agent. However, the dose of each agent was adjusted at the clinician’s discretion based on the objective of reaching target BP. Finally, being a large referral center, many of our patients were admitted from other institutions, and it is likely that the majority had received some other anti-hypertensive agent before or during transport, which might explain the relatively low mean BP at the start of the drip; but this would likely be comparable in both groups.
Conclusion
This is the first study assessing the efficacy and safety of clevidipine in patients with AAS. Our results suggest that clevidipine is as effective as SNP for the treatment of hypertension in the acute care of patients with AAS, and does not carry higher mortality or higher hypotension rates when compared to SNP. Our findings suggest that, in an era where the price of SNP has significantly increased, clevidipine is an efficacious, safe and cost-effective tool to treat patients with AAS. Further prospective, randomized studies are required to assess hard outcomes and cost-effectiveness analysis.
The authors would like to thank Li Qian from the department of Health Outcomes and Policy at the University of Florida, for the assistance with the statistical analysis for this manuscript.
None declared. The authors report no financial relationships or conflicts of interest regarding the content herein as well as no relationships to industry.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
Author notes
Both of these authors contributed equally to this work.




Comments