-
PDF
- Split View
-
Views
-
Cite
Cite
Pablo Jorge-Perez, Nikolaos Nikolaou, Katia Donadello, Abdo Khoury, Wilhelm Behringer, Christian Hassager, Bernd Boettiger, Alessandro Sionis, Jerry Nolan, Alain Combes, Tom Quinn, Susanna Price, Johannes Grand, Management of comatose survivors of out-of-hospital cardiac arrest in Europe: current treatment practice and adherence to guidelines. A joint survey by the Association for Acute CardioVascular Care (ACVC) of the ESC, the European Resuscitation Council (ERC), the European Society for Emergency Medicine (EUSEM), and the European Society of Intensive Care Medicine (ESICM), European Heart Journal. Acute Cardiovascular Care, Volume 12, Issue 2, February 2023, Pages 96–105, https://doi.org/10.1093/ehjacc/zuac153
Close - Share Icon Share
Abstract
International guidelines give recommendations for the management of comatose out-of-hospital cardiac arrest (OHCA) survivors. We aimed to investigate adherence to guidelines and disparities in the treatment of OHCA in hospitals in Europe.
A web-based, multi-institutional, multinational survey in Europe was conducted using an electronic platform with a predefined questionnaire developed by experts in post-resuscitation care. The survey was disseminated to all members of the societies via email, social media, websites, and newsletters in June 2021. Of 252 answers received, 237 responses from different units were included and 166 (70%) were from cardiac arrest centres. First-line vasopressor used was noradrenaline in 195 (83%) and the first-line inotrope was dobutamine in 148 (64%) of the responses. Echocardiography is available 24/7 in 204 (87%) institutions. Targeted temperature management was used in 160 (75%) institutions for adult comatose survivors of OHCA with an initial shockable rhythm. Invasive or external cooling methods with feedback were used in 72 cardiac arrest centres (44%) and 17 (24%) non-cardiac arrest centres (P < 0.0003). A target temperature between 32 and 34°C was preferred by 46 centres (21%); a target between 34 and 36°C by 103 centres (52%); and <37.5°C by 35 (16%). Multimodal neuroprognostication was poorly implemented and a follow-up at 3 months after discharge was done in 71 (30%) institutions.
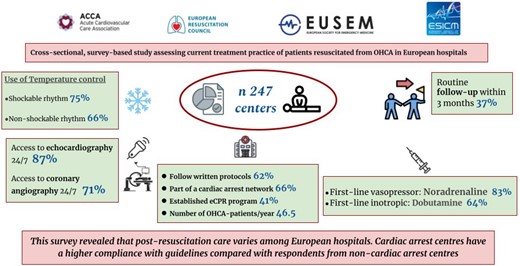
Post-resuscitation care is not well established and varies among centres in European hospitals. Cardiac arrest centres have a higher coherence with guidelines compared with respondents from non-cardiac arrest centres. The overall inconsistency in approaches and deviation from recommendations could be a focus for improvement.
In line with the Journal's conflict of interest policy, this paper was handled by Borja Ibanez.
Introduction
An increasing number of adults are resuscitated from out-of-hospital cardiac arrest (OHCA) and require post-resuscitation care.1,2 In Europe, ∼40–80 per 100 000 habitants per year have an OHCA with only approximately one person in 10 surviving hospital discharge.1–5 In the patients remaining comatose after resuscitation and who need intensive care, the mortality remains as high as 40–60%.5–7 In comatose survivors of OHCA, hypoxic–ischaemic brain injury is the primary cause of mortality and long-term neurological disability in survivors.8 The goal of post-resuscitation care is to support organ function and optimize oxygen delivery to the brain and ultimately improve outcomes.3
Early in-hospital interventions involve haemodynamic optimization and acute coronary angiography with percutaneous coronary intervention (PCI) in selected patients.9–12 General intensive care measures and temperature control (TC) are instigated to mitigate hypoxic–ischaemic brain injury6 and support organ function. This also involves controlling oxygen and carbon dioxide values during mechanical ventilation and controlling blood glucose values.13 Neurological prognostication is a vital part of post-resuscitation care to identify patients destined to have a poor outcome and to give clarity to relatives. Previous studies have suggested that the implementation of therapies and outcomes varies between different European countries and some interventions may be underused.14–17 However, because of the vast diversity in infrastructure and hospitals in Europe, these guideline recommendations might not be feasible in all areas. Treatment of patients with trauma, burns, or stroke in regional specialist centres is associated with improved outcomes and the same may apply to cardiac arrest patients treated in cardiac arrest centres.18,19
Objectives
The primary aim was to explore the current standard of post-resuscitation care throughout Europe. The secondary aim is to explore disparities in adherence to guidelines between cardiac arrest centres and non-cardiac arrest centres and, ultimately, to form a foundation for improved outcomes and quality of life of patients.
Methods
This is a cross-sectional, survey-based study assessing the current treatment practice of patients resuscitated from OHCA in European hospitals. The study was conducted by a task force initiated by the Association for Acute Cardiovascular Care (ACVC) of the European Society of Cardiology in collaboration with scientific societies of physicians treating comatose patients resuscitated from OHCA: European Resuscitation Council (ERC), European Society for Emergency Medicine (EUSEM) and European Society of Intensive Care Medicine (ESICM).
Objectives of the post-cardiac arrest management group
The survey was developed within the Post-Cardiac Arrest Management (POSTCAM) project. The overall objective of the project is to improve the quality of post-resuscitation care across Europe and to optimize patient care and outcomes. The project will conduct a gap analysis with the identification of adherence to guidelines in Europe and areas where the treatment of patients can be improved (Phase 1). Thereafter the development of international quality indicators (QIs), in line with the standards will be established, as has been done already nationally in Germany before.20 (Phase 2). Actions and tools will be developed for dissemination into countries to improve POSTCAM (Phase 3).
Based on existing scientific evidence and expert consensus, the objectives are to:
Explore the current standard of post-resuscitation care across Europe.
Reduce variation within and between countries and centres based on adherence to defined QIs.
Develop and set standards of reference and QIs to measure the improvement of post-resuscitation care.
Increase survival and improve neurological and quality of life outcomes of patients.
Improve post-resuscitation care of comatose, resuscitated OHCA patients.
Advocate for the need for hospital accreditation to reach or to implement standardization of care across Europe based on current international guidelines.
Raise awareness Europe-wide via the national societies across the disciplines involved
Design of the survey
The open and voluntary survey link was disseminated to all members of the involved societies via email, social media, websites, and newsletters on 1 June 2021 and closed on 31 July 2021.
The survey was designed using SurveyMonkey software (SurveyMonkey Inc., San Mateo, California, USA) containing 50 questions (Supplemental Digital Content 1, https://da.research.net/results/SM-YX8NQR3Y9/).
The survey questions comprised four categories—(i) cardiac arrest centres and prehospital care, (ii) TTM and early intensive care management, (iii) haemodynamic and acute coronary angiography, (iv) neuroprognostication and neurological follow-up post-discharge.
The survey included multiple-choice and open-ended (free text) questions. Cardiac arrest centres (CACs) were identified if centres fulfil specific criteria defined as (i) 24/7 availability of an on-site coronary angiography laboratory; (ii) an emergency department; (iii) an intensive care unit; (iv) imaging facilities such as echocardiography, computed tomography and magnetic resonance imaging; (v) and a protocol outlining the transfer of selected patients to cardiac arrest centres with additional resources 21 in order to achieve faster times to treatment and to be more compliant with guideline-recommended therapy.3 A draft was piloted by the task force leaders (J.G. and P.J.) and was discussed with the complete task force consisting of experts in post-resuscitation care. Following feedback, the survey underwent revision and further testing by the task force leaders. Through an online workshop by members with expertise in the field of post-resuscitation from all societies, the survey was once again revised and accepted for distribution.
The survey conformed to the Declaration of Helsinki’s ethical standards. Participation in the survey was voluntary, no incentives were offered for participation, no personal nor sensitive information was requested/collected, and all responses were anonymized. The task force considers this survey as a quality improvement project. No experimental, patient, or personal data were recorded or analysed. This was a survey among critical care physicians without the need for ethical approval by local ethics committees.
Data analysis
Data were exported from Survey Monkey software in a comma-separated value file format into Microsoft Excel for Mac (Washington, USA, Version 16.35). Categorical variables are presented as counts and percentages and differences were tested with the χ2 test or Fisher's exact test if expected counts were <five in the analysis. Continuous variables are presented as mean and standard deviation (±SD) if data were normally distributed and median and quartiles (q1–q3) for non-normal distributed data. Differences were tested with Mann–Whitney U test.
Data were divided into specialized centres characterized as cardiac arrest centres and non-cardiac arrest centres, based on previous consensus document.21 All completed questionnaires were computer-analysed, and the final data were double-checked before statistical analysis. Only one response per centre was included in the final analysis. Statistical analyses were performed using the SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
Results
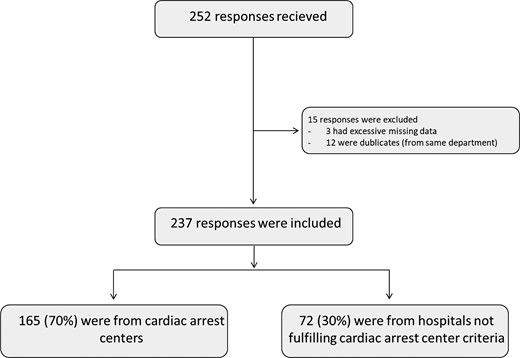
A total of 252 answers were received. Three responses were deleted due to extensive missing data, and after removing duplicates (n = 13), 237 responses from different units that admit comatose OHCA survivors were included for the analysis (Figure 1). Participants were representatives of different European societies involved in the post-resuscitation care of OHCA patients. The most common nationality was Spanish [32 (14%)], Greece [14 (6%)], Germany [13 (5,5%)], and the UK [13 (5,5%)] (see Supplementary material online, Table S1). Responders who are active in European societies (ACVC, ERC, ESICM, EUSEM), with non-European nationalities were accepted. Transnational differences within EU countries were also analyzed (see Supplementary material online, Table S2). Of all responses, 166 (70%) were from CACs, as shown in Table 1. Written protocols for the management of cardiac arrest patients are available and followed in 148 (63%) cases and 156 (66%) are part of a cardiac arrest network (receiving admissions from EMS (Emergency Medical Services) directly bypassing other hospitals or/and receiving patients from other hospitals). The median number of patients admitted per unit per year was 46.5 (quartiles 19–75). In cardiac arrest centres, the median was 50 (quartiles: 22–85) and 25 (quartiles: 6–51) in non-cardiac arrest centres, P < 0.0001 (Figure 2). A cardiac origin of the arrest was the most common cause with 126 (55%) being due to ST elevation myocardial infarction (STEMI) and 27% caused by non-STEMI (NSTEMI). An extracorporeal cardiopulmonary resuscitation (eCPR) programme coordinated with prehospital systems to receive patients with refractory OHCA existed in 97 (40%).
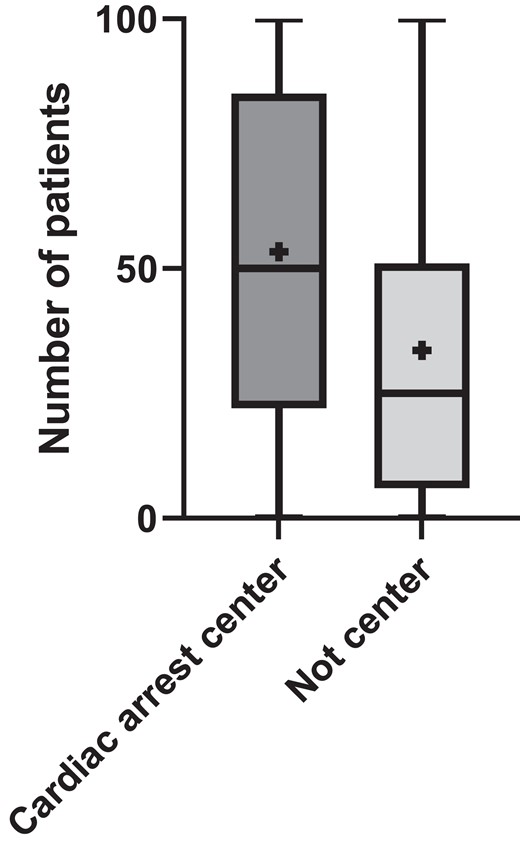
Box plot of number of patients per unit per year. Horizontal line indicates median. + indicates mean. Rectangular box covers 25 and 75 quartiles, whereas whiskers indicate minimum and maximum values.
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Type of unit (more than one answer possible): | ||||
| ȃGeneral intensive care | 96 (41%) | 57 (35%) | 39 (54%) | 0.007 |
| ȃCardiac intensive care | 91 (38%) | 78 (47%) | 13 (18%) | <0.001 |
| ȃEmergency department | 62 (26%) | 40 (24%) | 22 (31%) | 0.33 |
| ȃCardio-thoracic intensive care | 16 (7%) | 16 (10%) | 0 | 0.006 |
| Characteristics of the department | ||||
| Follow written protocols for OHCA management | 148 (62%) | 113 (68%) | 35 (49%) | 0.01 |
| Part of a cardiac arrest networka | 156 (66%) | 123 (75%) | 33 (46%) | <0.001 |
| eCPR programme | 97 (41%) | 90 (55%) | 7 (10%) | <0.001 |
| Number of comatose OHCA patients/year | 46.5 (19–75) | 50 (22–85) | 25 (6–51) | <0.001 |
| Most frequent type of OHCA: | ||||
| ȃSTEMI | 126 (55%) | 100 (62%) | 26 (38%) | <0.001 |
| ȃCardiac cause without STEMI | 63 (27%) | 44 (27%) | 19 (28%) | <0.001 |
| ȃNon-cardiac cause | 41 (18%) | 18 (11%) | 23 (34%) | <0.001 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Type of unit (more than one answer possible): | ||||
| ȃGeneral intensive care | 96 (41%) | 57 (35%) | 39 (54%) | 0.007 |
| ȃCardiac intensive care | 91 (38%) | 78 (47%) | 13 (18%) | <0.001 |
| ȃEmergency department | 62 (26%) | 40 (24%) | 22 (31%) | 0.33 |
| ȃCardio-thoracic intensive care | 16 (7%) | 16 (10%) | 0 | 0.006 |
| Characteristics of the department | ||||
| Follow written protocols for OHCA management | 148 (62%) | 113 (68%) | 35 (49%) | 0.01 |
| Part of a cardiac arrest networka | 156 (66%) | 123 (75%) | 33 (46%) | <0.001 |
| eCPR programme | 97 (41%) | 90 (55%) | 7 (10%) | <0.001 |
| Number of comatose OHCA patients/year | 46.5 (19–75) | 50 (22–85) | 25 (6–51) | <0.001 |
| Most frequent type of OHCA: | ||||
| ȃSTEMI | 126 (55%) | 100 (62%) | 26 (38%) | <0.001 |
| ȃCardiac cause without STEMI | 63 (27%) | 44 (27%) | 19 (28%) | <0.001 |
| ȃNon-cardiac cause | 41 (18%) | 18 (11%) | 23 (34%) | <0.001 |
Defined as: receiving admissions from EMS directly bypassing other hospitals or/and receiving patients from other hospital
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Type of unit (more than one answer possible): | ||||
| ȃGeneral intensive care | 96 (41%) | 57 (35%) | 39 (54%) | 0.007 |
| ȃCardiac intensive care | 91 (38%) | 78 (47%) | 13 (18%) | <0.001 |
| ȃEmergency department | 62 (26%) | 40 (24%) | 22 (31%) | 0.33 |
| ȃCardio-thoracic intensive care | 16 (7%) | 16 (10%) | 0 | 0.006 |
| Characteristics of the department | ||||
| Follow written protocols for OHCA management | 148 (62%) | 113 (68%) | 35 (49%) | 0.01 |
| Part of a cardiac arrest networka | 156 (66%) | 123 (75%) | 33 (46%) | <0.001 |
| eCPR programme | 97 (41%) | 90 (55%) | 7 (10%) | <0.001 |
| Number of comatose OHCA patients/year | 46.5 (19–75) | 50 (22–85) | 25 (6–51) | <0.001 |
| Most frequent type of OHCA: | ||||
| ȃSTEMI | 126 (55%) | 100 (62%) | 26 (38%) | <0.001 |
| ȃCardiac cause without STEMI | 63 (27%) | 44 (27%) | 19 (28%) | <0.001 |
| ȃNon-cardiac cause | 41 (18%) | 18 (11%) | 23 (34%) | <0.001 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Type of unit (more than one answer possible): | ||||
| ȃGeneral intensive care | 96 (41%) | 57 (35%) | 39 (54%) | 0.007 |
| ȃCardiac intensive care | 91 (38%) | 78 (47%) | 13 (18%) | <0.001 |
| ȃEmergency department | 62 (26%) | 40 (24%) | 22 (31%) | 0.33 |
| ȃCardio-thoracic intensive care | 16 (7%) | 16 (10%) | 0 | 0.006 |
| Characteristics of the department | ||||
| Follow written protocols for OHCA management | 148 (62%) | 113 (68%) | 35 (49%) | 0.01 |
| Part of a cardiac arrest networka | 156 (66%) | 123 (75%) | 33 (46%) | <0.001 |
| eCPR programme | 97 (41%) | 90 (55%) | 7 (10%) | <0.001 |
| Number of comatose OHCA patients/year | 46.5 (19–75) | 50 (22–85) | 25 (6–51) | <0.001 |
| Most frequent type of OHCA: | ||||
| ȃSTEMI | 126 (55%) | 100 (62%) | 26 (38%) | <0.001 |
| ȃCardiac cause without STEMI | 63 (27%) | 44 (27%) | 19 (28%) | <0.001 |
| ȃNon-cardiac cause | 41 (18%) | 18 (11%) | 23 (34%) | <0.001 |
Defined as: receiving admissions from EMS directly bypassing other hospitals or/and receiving patients from other hospital
Immediate intensive care management
The first-line vasopressor was noradrenaline in 195 (83%) and the first-line inotrope was dobutamine in 148 (64%) of the cases. The target mean arterial pressure was >65 mmHg in 121 (52%) of the participant´s centres. Cardiac output (CO) was measured routinely in 38% of the centres, but 52% measured CO only in selected patients (methods for CO assessment are shown in Table 2). First echocardiography is performed on admission in 77% of the patients and 19% within the first 24 h. Two hundred and four (87%) participants have access to echocardiography 24/7. The availability of mechanical support devices is variable (Table 2), intra-aortic balloon pump (IABP) being the most common available device (136, 57%), followed by VA-ECMO and Impella devices. One hundred and eighty (72%) centres have 24/7 access to coronary angiography. In patients with STEMI, emergent coronary angiography is performed in 197 (79%) patients with STEMI and in 107 (45%) NSTEMI only when high suspicion of acute MI has been identified.
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Diagnostic possibilities | ||||
| ȃAccess to echocardiography 24/7 | 204 (87%) | 158 (96%) | 46 (65%) | <0.001 |
| ȃAccess to coronary angiography 24/7 | 165 (71%) | 154 (94%) | 11 (15%) | <0.001 |
| Methods used method for cardiac output monitoring | ||||
| ȃDoppler echocardiography | 136 (57%) | 104 (63%) | 32 (44%) | 0.008 |
| ȃPICCO | 136 (57%) | 104 (63%) | 32 (44%) | 0.007 |
| ȃPulmonary artery catheter | 59 (24%) | 56 (34%) | 3 (4%) | <0.001 |
| ȃNever measured | 26 (11%) | 12 (7%) | 14 (19%) | 0.006 |
| First-line vasopressor: | ||||
| ȃNoradrenaline | 195 (83%) | 140 (85%) | 55 (79%) | 0.17 |
| ȃAdrenaline | 32 (14%) | 19 (12%) | 13 (19%) | 0.16 |
| ȃDopamine | 4 (2%) | 3 (2%) | 1 (1%) | 0.81 |
| ȃother | 5 (2%) | 3 (2%) | 2 (2%) | 0.75 |
| First-line inotropic drug if indicated: | ||||
| ȃDobutamine | 148 (64%) | 109 (67%) | 39 (56%) | 0.24 |
| ȃAdrenaline | 40 (17%) | 23 (14%) | 17 (24%) | 0.14 |
| ȃMilrinone | 14 (6%) | 12 (7%) | 2 (3%) | 0.28 |
| ȃDopamine | 13 (6%) | 10 (6%) | 3 (4%) | 0.19 |
| ȃLevosimendan | 11 (5%) | 5 (3%) | 6 (9%) | 0.34 |
| ȃOther | 7 (3%) | 4 (2%) | 3 (4%) | 0.87 |
| Lower acceptable level of mean arterial pressure | ||||
| ȃ > 55 mmHg | 7 (3%) | 5 (3%) | 2 (3%) | |
| ȃ > 60 mmHg | 39 (17%) | 29 (18%) | 10 (14%) | |
| ȃ > 65 mmHg | 121 (52%) | 84 (52%) | 37 (53%) | |
| ȃ > 70 mmHg | 31 (14%) | 18 (11%) | 13 (19%) | |
| ȃ > 75 mmHg | 10 (4%) | 8 (5%) | 2 (3%) | |
| ȃNo level for blood pressure | 24 (10%) | 18 (11%) | 6 (9%) | |
| Type of mechanical circulatory support device when indicated | ||||
| ȃIABP | 136 (57%) | 122 (74%) | 14 (19%) | <0.001 |
| ȃECMO | 105 (44%) | 99 (60%) | 6 (8%) | <0.001 |
| ȃImpella | 76 (32%) | 74 (45%) | 2 (3%) | <0.001 |
| ȃNot possible | 71 (30%) | 19 (12%) | 52 (72%) | <0.001 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Diagnostic possibilities | ||||
| ȃAccess to echocardiography 24/7 | 204 (87%) | 158 (96%) | 46 (65%) | <0.001 |
| ȃAccess to coronary angiography 24/7 | 165 (71%) | 154 (94%) | 11 (15%) | <0.001 |
| Methods used method for cardiac output monitoring | ||||
| ȃDoppler echocardiography | 136 (57%) | 104 (63%) | 32 (44%) | 0.008 |
| ȃPICCO | 136 (57%) | 104 (63%) | 32 (44%) | 0.007 |
| ȃPulmonary artery catheter | 59 (24%) | 56 (34%) | 3 (4%) | <0.001 |
| ȃNever measured | 26 (11%) | 12 (7%) | 14 (19%) | 0.006 |
| First-line vasopressor: | ||||
| ȃNoradrenaline | 195 (83%) | 140 (85%) | 55 (79%) | 0.17 |
| ȃAdrenaline | 32 (14%) | 19 (12%) | 13 (19%) | 0.16 |
| ȃDopamine | 4 (2%) | 3 (2%) | 1 (1%) | 0.81 |
| ȃother | 5 (2%) | 3 (2%) | 2 (2%) | 0.75 |
| First-line inotropic drug if indicated: | ||||
| ȃDobutamine | 148 (64%) | 109 (67%) | 39 (56%) | 0.24 |
| ȃAdrenaline | 40 (17%) | 23 (14%) | 17 (24%) | 0.14 |
| ȃMilrinone | 14 (6%) | 12 (7%) | 2 (3%) | 0.28 |
| ȃDopamine | 13 (6%) | 10 (6%) | 3 (4%) | 0.19 |
| ȃLevosimendan | 11 (5%) | 5 (3%) | 6 (9%) | 0.34 |
| ȃOther | 7 (3%) | 4 (2%) | 3 (4%) | 0.87 |
| Lower acceptable level of mean arterial pressure | ||||
| ȃ > 55 mmHg | 7 (3%) | 5 (3%) | 2 (3%) | |
| ȃ > 60 mmHg | 39 (17%) | 29 (18%) | 10 (14%) | |
| ȃ > 65 mmHg | 121 (52%) | 84 (52%) | 37 (53%) | |
| ȃ > 70 mmHg | 31 (14%) | 18 (11%) | 13 (19%) | |
| ȃ > 75 mmHg | 10 (4%) | 8 (5%) | 2 (3%) | |
| ȃNo level for blood pressure | 24 (10%) | 18 (11%) | 6 (9%) | |
| Type of mechanical circulatory support device when indicated | ||||
| ȃIABP | 136 (57%) | 122 (74%) | 14 (19%) | <0.001 |
| ȃECMO | 105 (44%) | 99 (60%) | 6 (8%) | <0.001 |
| ȃImpella | 76 (32%) | 74 (45%) | 2 (3%) | <0.001 |
| ȃNot possible | 71 (30%) | 19 (12%) | 52 (72%) | <0.001 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Diagnostic possibilities | ||||
| ȃAccess to echocardiography 24/7 | 204 (87%) | 158 (96%) | 46 (65%) | <0.001 |
| ȃAccess to coronary angiography 24/7 | 165 (71%) | 154 (94%) | 11 (15%) | <0.001 |
| Methods used method for cardiac output monitoring | ||||
| ȃDoppler echocardiography | 136 (57%) | 104 (63%) | 32 (44%) | 0.008 |
| ȃPICCO | 136 (57%) | 104 (63%) | 32 (44%) | 0.007 |
| ȃPulmonary artery catheter | 59 (24%) | 56 (34%) | 3 (4%) | <0.001 |
| ȃNever measured | 26 (11%) | 12 (7%) | 14 (19%) | 0.006 |
| First-line vasopressor: | ||||
| ȃNoradrenaline | 195 (83%) | 140 (85%) | 55 (79%) | 0.17 |
| ȃAdrenaline | 32 (14%) | 19 (12%) | 13 (19%) | 0.16 |
| ȃDopamine | 4 (2%) | 3 (2%) | 1 (1%) | 0.81 |
| ȃother | 5 (2%) | 3 (2%) | 2 (2%) | 0.75 |
| First-line inotropic drug if indicated: | ||||
| ȃDobutamine | 148 (64%) | 109 (67%) | 39 (56%) | 0.24 |
| ȃAdrenaline | 40 (17%) | 23 (14%) | 17 (24%) | 0.14 |
| ȃMilrinone | 14 (6%) | 12 (7%) | 2 (3%) | 0.28 |
| ȃDopamine | 13 (6%) | 10 (6%) | 3 (4%) | 0.19 |
| ȃLevosimendan | 11 (5%) | 5 (3%) | 6 (9%) | 0.34 |
| ȃOther | 7 (3%) | 4 (2%) | 3 (4%) | 0.87 |
| Lower acceptable level of mean arterial pressure | ||||
| ȃ > 55 mmHg | 7 (3%) | 5 (3%) | 2 (3%) | |
| ȃ > 60 mmHg | 39 (17%) | 29 (18%) | 10 (14%) | |
| ȃ > 65 mmHg | 121 (52%) | 84 (52%) | 37 (53%) | |
| ȃ > 70 mmHg | 31 (14%) | 18 (11%) | 13 (19%) | |
| ȃ > 75 mmHg | 10 (4%) | 8 (5%) | 2 (3%) | |
| ȃNo level for blood pressure | 24 (10%) | 18 (11%) | 6 (9%) | |
| Type of mechanical circulatory support device when indicated | ||||
| ȃIABP | 136 (57%) | 122 (74%) | 14 (19%) | <0.001 |
| ȃECMO | 105 (44%) | 99 (60%) | 6 (8%) | <0.001 |
| ȃImpella | 76 (32%) | 74 (45%) | 2 (3%) | <0.001 |
| ȃNot possible | 71 (30%) | 19 (12%) | 52 (72%) | <0.001 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Diagnostic possibilities | ||||
| ȃAccess to echocardiography 24/7 | 204 (87%) | 158 (96%) | 46 (65%) | <0.001 |
| ȃAccess to coronary angiography 24/7 | 165 (71%) | 154 (94%) | 11 (15%) | <0.001 |
| Methods used method for cardiac output monitoring | ||||
| ȃDoppler echocardiography | 136 (57%) | 104 (63%) | 32 (44%) | 0.008 |
| ȃPICCO | 136 (57%) | 104 (63%) | 32 (44%) | 0.007 |
| ȃPulmonary artery catheter | 59 (24%) | 56 (34%) | 3 (4%) | <0.001 |
| ȃNever measured | 26 (11%) | 12 (7%) | 14 (19%) | 0.006 |
| First-line vasopressor: | ||||
| ȃNoradrenaline | 195 (83%) | 140 (85%) | 55 (79%) | 0.17 |
| ȃAdrenaline | 32 (14%) | 19 (12%) | 13 (19%) | 0.16 |
| ȃDopamine | 4 (2%) | 3 (2%) | 1 (1%) | 0.81 |
| ȃother | 5 (2%) | 3 (2%) | 2 (2%) | 0.75 |
| First-line inotropic drug if indicated: | ||||
| ȃDobutamine | 148 (64%) | 109 (67%) | 39 (56%) | 0.24 |
| ȃAdrenaline | 40 (17%) | 23 (14%) | 17 (24%) | 0.14 |
| ȃMilrinone | 14 (6%) | 12 (7%) | 2 (3%) | 0.28 |
| ȃDopamine | 13 (6%) | 10 (6%) | 3 (4%) | 0.19 |
| ȃLevosimendan | 11 (5%) | 5 (3%) | 6 (9%) | 0.34 |
| ȃOther | 7 (3%) | 4 (2%) | 3 (4%) | 0.87 |
| Lower acceptable level of mean arterial pressure | ||||
| ȃ > 55 mmHg | 7 (3%) | 5 (3%) | 2 (3%) | |
| ȃ > 60 mmHg | 39 (17%) | 29 (18%) | 10 (14%) | |
| ȃ > 65 mmHg | 121 (52%) | 84 (52%) | 37 (53%) | |
| ȃ > 70 mmHg | 31 (14%) | 18 (11%) | 13 (19%) | |
| ȃ > 75 mmHg | 10 (4%) | 8 (5%) | 2 (3%) | |
| ȃNo level for blood pressure | 24 (10%) | 18 (11%) | 6 (9%) | |
| Type of mechanical circulatory support device when indicated | ||||
| ȃIABP | 136 (57%) | 122 (74%) | 14 (19%) | <0.001 |
| ȃECMO | 105 (44%) | 99 (60%) | 6 (8%) | <0.001 |
| ȃImpella | 76 (32%) | 74 (45%) | 2 (3%) | <0.001 |
| ȃNot possible | 71 (30%) | 19 (12%) | 52 (72%) | <0.001 |
Routine administration of steroids, seizure, or antibiotics prophylaxis was infrequent with 11 (5%), 30 (10%), and 65 (30%), respectively, and with no differences between CAC and other centres.
Temperature control
TC was used in 160 centres (75%) in adult comatose survivors of OHCA with an initial shockable rhythm and 142 (66%) with non-shockable rhythm, starting in 65% of the centres in the ICU, with statistically significant more frequent use in CACs, following either shockable (P < 0.006) and non-shockable rhythm (P < 0.06). Invasive or external methods with feedback are used in 72 CACs (44%) and in 17 (24%) non-CACs (Table 3). The target temperature was very variable: a target between 32 and 34°C was preferred by 46 centres (21%); a target between 34 and 36°C by 103 centres (52%); and a < 37.5°C objective is selected by 35 (16%) participants (Figure 3). Duration of TC after rewarming was very variable with 64 (31%) centres aiming for 72 h and with no significant differences between CACs and non-CACs as shown in Table 3.
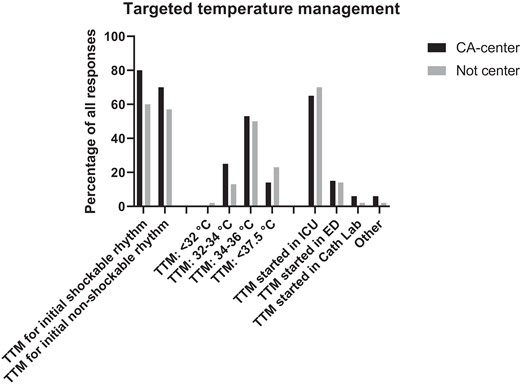
Temperature control practices following OHCA. TTM, targeted temperature management; ICU, intensive care unit; ED, Emergency Department; CA centre, cardiac arrest centre.
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Prophylactic drugs | ||||
| Routinely administration of steroids | 11 (5%) | 7 (5%) | 4 (7%) | 0.52 |
| Routinely administration of seizure prophylaxis | 30 (10%) | 16 (12%) | 4 (7%) | 0.38 |
| Routinely administration of prophylactic antibiotics: | 65 (30%) | 47 (30%) | 18 (30%) | 0.94 |
| Targeted temperature management | ||||
| ȃroutinely use of TTM for initial shockable rhythm | 160 (75%) | 123 (80%) | 37 (60%) | 0.006 |
| ȃroutinely use of TTM for initial non-shockable rhythm | 142 (66%) | 108 (70%) | 34 (57%) | 0.06 |
| Place of TTM induction | ||||
| ȃICU | 141 (66%) | 100 (65%) | 41 (70%) | 0.43 |
| ȃEmergency Department | 32 (15%) | 24 (15%) | 8 (14%) | |
| ȃCath lab | 10 (5%) | 9 (6%) | 1 (2%) | |
| ȃOther | 10 (4%) | 9 (6%) | 1 (2%) | |
| TTM-methods (more than one is possible) | ||||
| ȃAntipyretic medication | 104 (44%) | 69 (42%) | 35 (49%) | 0.33 |
| ȃCold fluids | 99 (42%) | 69 (42%) | 30 (42%) | 0.98 |
| ȃExternal cooling without feedback | 109 (46%) | 71 (43%) | 38 (53%) | 0.25 |
| ȃInvasive and external cooling with feedback | 89 (38%) | 72 (44%) | 17 (24%) | 0.0003 |
| Level of target temperature | ||||
| ȃ < 32°C | 1 (0.5%) | 0 (0%) | 1 (2%) | |
| ȃbetween 32–34°C | 46 (21%) | 38 (25%) | 8 (13%) | 0.04 |
| ȃBetween 34–36°C | 103 (52%) | 82 (53%) | 31 (50%) | |
| ȃ < 37.5°C | 35 (16%) | 21 (14%) | 14 (23%) | |
| Duration of TTM including fever control after cooling | ||||
| ȃ24 h in total from start of TTM | 28 (14%) | 20 (14%) | 8 (14%) | |
| ȃ48 h in total from start of TTM | 29 (14%) | 24 (16%) | 5 (9%) | |
| ȃ72 h in total from start of TTM | 64 (31%) | 49 (33%) | 15 (26%) | 0.31 |
| ȃMore than 72 h if patient has fever | 25 (12%) | 18 (12%) | 7 (26%) | |
| ȃOther | 60 (29%) | 37 (25%) | 23 (40%) |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Prophylactic drugs | ||||
| Routinely administration of steroids | 11 (5%) | 7 (5%) | 4 (7%) | 0.52 |
| Routinely administration of seizure prophylaxis | 30 (10%) | 16 (12%) | 4 (7%) | 0.38 |
| Routinely administration of prophylactic antibiotics: | 65 (30%) | 47 (30%) | 18 (30%) | 0.94 |
| Targeted temperature management | ||||
| ȃroutinely use of TTM for initial shockable rhythm | 160 (75%) | 123 (80%) | 37 (60%) | 0.006 |
| ȃroutinely use of TTM for initial non-shockable rhythm | 142 (66%) | 108 (70%) | 34 (57%) | 0.06 |
| Place of TTM induction | ||||
| ȃICU | 141 (66%) | 100 (65%) | 41 (70%) | 0.43 |
| ȃEmergency Department | 32 (15%) | 24 (15%) | 8 (14%) | |
| ȃCath lab | 10 (5%) | 9 (6%) | 1 (2%) | |
| ȃOther | 10 (4%) | 9 (6%) | 1 (2%) | |
| TTM-methods (more than one is possible) | ||||
| ȃAntipyretic medication | 104 (44%) | 69 (42%) | 35 (49%) | 0.33 |
| ȃCold fluids | 99 (42%) | 69 (42%) | 30 (42%) | 0.98 |
| ȃExternal cooling without feedback | 109 (46%) | 71 (43%) | 38 (53%) | 0.25 |
| ȃInvasive and external cooling with feedback | 89 (38%) | 72 (44%) | 17 (24%) | 0.0003 |
| Level of target temperature | ||||
| ȃ < 32°C | 1 (0.5%) | 0 (0%) | 1 (2%) | |
| ȃbetween 32–34°C | 46 (21%) | 38 (25%) | 8 (13%) | 0.04 |
| ȃBetween 34–36°C | 103 (52%) | 82 (53%) | 31 (50%) | |
| ȃ < 37.5°C | 35 (16%) | 21 (14%) | 14 (23%) | |
| Duration of TTM including fever control after cooling | ||||
| ȃ24 h in total from start of TTM | 28 (14%) | 20 (14%) | 8 (14%) | |
| ȃ48 h in total from start of TTM | 29 (14%) | 24 (16%) | 5 (9%) | |
| ȃ72 h in total from start of TTM | 64 (31%) | 49 (33%) | 15 (26%) | 0.31 |
| ȃMore than 72 h if patient has fever | 25 (12%) | 18 (12%) | 7 (26%) | |
| ȃOther | 60 (29%) | 37 (25%) | 23 (40%) |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Prophylactic drugs | ||||
| Routinely administration of steroids | 11 (5%) | 7 (5%) | 4 (7%) | 0.52 |
| Routinely administration of seizure prophylaxis | 30 (10%) | 16 (12%) | 4 (7%) | 0.38 |
| Routinely administration of prophylactic antibiotics: | 65 (30%) | 47 (30%) | 18 (30%) | 0.94 |
| Targeted temperature management | ||||
| ȃroutinely use of TTM for initial shockable rhythm | 160 (75%) | 123 (80%) | 37 (60%) | 0.006 |
| ȃroutinely use of TTM for initial non-shockable rhythm | 142 (66%) | 108 (70%) | 34 (57%) | 0.06 |
| Place of TTM induction | ||||
| ȃICU | 141 (66%) | 100 (65%) | 41 (70%) | 0.43 |
| ȃEmergency Department | 32 (15%) | 24 (15%) | 8 (14%) | |
| ȃCath lab | 10 (5%) | 9 (6%) | 1 (2%) | |
| ȃOther | 10 (4%) | 9 (6%) | 1 (2%) | |
| TTM-methods (more than one is possible) | ||||
| ȃAntipyretic medication | 104 (44%) | 69 (42%) | 35 (49%) | 0.33 |
| ȃCold fluids | 99 (42%) | 69 (42%) | 30 (42%) | 0.98 |
| ȃExternal cooling without feedback | 109 (46%) | 71 (43%) | 38 (53%) | 0.25 |
| ȃInvasive and external cooling with feedback | 89 (38%) | 72 (44%) | 17 (24%) | 0.0003 |
| Level of target temperature | ||||
| ȃ < 32°C | 1 (0.5%) | 0 (0%) | 1 (2%) | |
| ȃbetween 32–34°C | 46 (21%) | 38 (25%) | 8 (13%) | 0.04 |
| ȃBetween 34–36°C | 103 (52%) | 82 (53%) | 31 (50%) | |
| ȃ < 37.5°C | 35 (16%) | 21 (14%) | 14 (23%) | |
| Duration of TTM including fever control after cooling | ||||
| ȃ24 h in total from start of TTM | 28 (14%) | 20 (14%) | 8 (14%) | |
| ȃ48 h in total from start of TTM | 29 (14%) | 24 (16%) | 5 (9%) | |
| ȃ72 h in total from start of TTM | 64 (31%) | 49 (33%) | 15 (26%) | 0.31 |
| ȃMore than 72 h if patient has fever | 25 (12%) | 18 (12%) | 7 (26%) | |
| ȃOther | 60 (29%) | 37 (25%) | 23 (40%) |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Prophylactic drugs | ||||
| Routinely administration of steroids | 11 (5%) | 7 (5%) | 4 (7%) | 0.52 |
| Routinely administration of seizure prophylaxis | 30 (10%) | 16 (12%) | 4 (7%) | 0.38 |
| Routinely administration of prophylactic antibiotics: | 65 (30%) | 47 (30%) | 18 (30%) | 0.94 |
| Targeted temperature management | ||||
| ȃroutinely use of TTM for initial shockable rhythm | 160 (75%) | 123 (80%) | 37 (60%) | 0.006 |
| ȃroutinely use of TTM for initial non-shockable rhythm | 142 (66%) | 108 (70%) | 34 (57%) | 0.06 |
| Place of TTM induction | ||||
| ȃICU | 141 (66%) | 100 (65%) | 41 (70%) | 0.43 |
| ȃEmergency Department | 32 (15%) | 24 (15%) | 8 (14%) | |
| ȃCath lab | 10 (5%) | 9 (6%) | 1 (2%) | |
| ȃOther | 10 (4%) | 9 (6%) | 1 (2%) | |
| TTM-methods (more than one is possible) | ||||
| ȃAntipyretic medication | 104 (44%) | 69 (42%) | 35 (49%) | 0.33 |
| ȃCold fluids | 99 (42%) | 69 (42%) | 30 (42%) | 0.98 |
| ȃExternal cooling without feedback | 109 (46%) | 71 (43%) | 38 (53%) | 0.25 |
| ȃInvasive and external cooling with feedback | 89 (38%) | 72 (44%) | 17 (24%) | 0.0003 |
| Level of target temperature | ||||
| ȃ < 32°C | 1 (0.5%) | 0 (0%) | 1 (2%) | |
| ȃbetween 32–34°C | 46 (21%) | 38 (25%) | 8 (13%) | 0.04 |
| ȃBetween 34–36°C | 103 (52%) | 82 (53%) | 31 (50%) | |
| ȃ < 37.5°C | 35 (16%) | 21 (14%) | 14 (23%) | |
| Duration of TTM including fever control after cooling | ||||
| ȃ24 h in total from start of TTM | 28 (14%) | 20 (14%) | 8 (14%) | |
| ȃ48 h in total from start of TTM | 29 (14%) | 24 (16%) | 5 (9%) | |
| ȃ72 h in total from start of TTM | 64 (31%) | 49 (33%) | 15 (26%) | 0.31 |
| ȃMore than 72 h if patient has fever | 25 (12%) | 18 (12%) | 7 (26%) | |
| ȃOther | 60 (29%) | 37 (25%) | 23 (40%) |
Neuroprognostication
A standardized protocol for neurological examination was performed by 188 (79%) centres, increasing to 84% in CAC, with 18% using quantitative pupillometry. Brain CT scan is the most common head imaging test (169, 71% of the participants) and MRI is used in 46% of the centres. The first CT brain scan is performed always on admission in 28% of the centres and 45% only if no clear cause of cardiac arrest has been identified. Neuron-specific enolase (NSE) is the preferred biomarker used in 166 (49%) centres with increasing use in CACs (56% vs. 33% in non-CACs, P 0002). Intermittent electroencephalography (EEG) is the most common neurophysiological test and is performed in 173 (73%) participants, and somatosensory-evoked potentials (SSEPs) are used in 102 (42%) centres. All different variables analysed for neuroprognostication are more often performed in CACs, except the use of quantitative pupillometry (Table 4).
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Methods are used for prognostication | ||||
| ȃNeurological examination | 188 (79%) | 138 (84%) | 50 (69%) | 0.01 |
| ȃBrain CT | 169 (71%) | 123 (75%) | 46 (64%) | 0.09 |
| ȃBrain MRI | 108 (46%) | 82 (50%) | 26 (36%) | 0.05 |
| ȃQuantitative pupillometry | 42 (18%) | 30 (18%) | 12 (17%) | 0.78 |
| ȃNSE | 116 (49%) | 92 (56%) | 24 (33%) | 0.002 |
| ȃEEG | 173 (73%) | 130 (79%) | 43 (60%) | 0.002 |
| ȃSSEP | 102 (42%) | 77 (47%) | 25 (35%) | 0.09 |
| ȃBispectral index | 70 (30%) | 56 (34%) | 14 (19%) | 0.02 |
| Routine follow-up of all survivors within 3 months | 71 (37%) | 55 (40%) | 16 (30%) | 0.19 |
| Screen for cognitive challenges within 3 months | 66 (73%) | 53 (80%) | 13 (54%) | 0.01 |
| Screen for emotional challenges and fatigue within 3 months | 59 (65%) | 46 (70%) | 13 (52%) | 0.11 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Methods are used for prognostication | ||||
| ȃNeurological examination | 188 (79%) | 138 (84%) | 50 (69%) | 0.01 |
| ȃBrain CT | 169 (71%) | 123 (75%) | 46 (64%) | 0.09 |
| ȃBrain MRI | 108 (46%) | 82 (50%) | 26 (36%) | 0.05 |
| ȃQuantitative pupillometry | 42 (18%) | 30 (18%) | 12 (17%) | 0.78 |
| ȃNSE | 116 (49%) | 92 (56%) | 24 (33%) | 0.002 |
| ȃEEG | 173 (73%) | 130 (79%) | 43 (60%) | 0.002 |
| ȃSSEP | 102 (42%) | 77 (47%) | 25 (35%) | 0.09 |
| ȃBispectral index | 70 (30%) | 56 (34%) | 14 (19%) | 0.02 |
| Routine follow-up of all survivors within 3 months | 71 (37%) | 55 (40%) | 16 (30%) | 0.19 |
| Screen for cognitive challenges within 3 months | 66 (73%) | 53 (80%) | 13 (54%) | 0.01 |
| Screen for emotional challenges and fatigue within 3 months | 59 (65%) | 46 (70%) | 13 (52%) | 0.11 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Methods are used for prognostication | ||||
| ȃNeurological examination | 188 (79%) | 138 (84%) | 50 (69%) | 0.01 |
| ȃBrain CT | 169 (71%) | 123 (75%) | 46 (64%) | 0.09 |
| ȃBrain MRI | 108 (46%) | 82 (50%) | 26 (36%) | 0.05 |
| ȃQuantitative pupillometry | 42 (18%) | 30 (18%) | 12 (17%) | 0.78 |
| ȃNSE | 116 (49%) | 92 (56%) | 24 (33%) | 0.002 |
| ȃEEG | 173 (73%) | 130 (79%) | 43 (60%) | 0.002 |
| ȃSSEP | 102 (42%) | 77 (47%) | 25 (35%) | 0.09 |
| ȃBispectral index | 70 (30%) | 56 (34%) | 14 (19%) | 0.02 |
| Routine follow-up of all survivors within 3 months | 71 (37%) | 55 (40%) | 16 (30%) | 0.19 |
| Screen for cognitive challenges within 3 months | 66 (73%) | 53 (80%) | 13 (54%) | 0.01 |
| Screen for emotional challenges and fatigue within 3 months | 59 (65%) | 46 (70%) | 13 (52%) | 0.11 |
| . | Overall n = 237 . | Cardiac arrest centre n = 165 (70%) . | Not cardiac arrest centre n = 72 (30%) . | P-value . |
|---|---|---|---|---|
| Methods are used for prognostication | ||||
| ȃNeurological examination | 188 (79%) | 138 (84%) | 50 (69%) | 0.01 |
| ȃBrain CT | 169 (71%) | 123 (75%) | 46 (64%) | 0.09 |
| ȃBrain MRI | 108 (46%) | 82 (50%) | 26 (36%) | 0.05 |
| ȃQuantitative pupillometry | 42 (18%) | 30 (18%) | 12 (17%) | 0.78 |
| ȃNSE | 116 (49%) | 92 (56%) | 24 (33%) | 0.002 |
| ȃEEG | 173 (73%) | 130 (79%) | 43 (60%) | 0.002 |
| ȃSSEP | 102 (42%) | 77 (47%) | 25 (35%) | 0.09 |
| ȃBispectral index | 70 (30%) | 56 (34%) | 14 (19%) | 0.02 |
| Routine follow-up of all survivors within 3 months | 71 (37%) | 55 (40%) | 16 (30%) | 0.19 |
| Screen for cognitive challenges within 3 months | 66 (73%) | 53 (80%) | 13 (54%) | 0.01 |
| Screen for emotional challenges and fatigue within 3 months | 59 (65%) | 46 (70%) | 13 (52%) | 0.11 |
Organ donation in cases of brain death is considered in 208 (88%) centres and 69% of the centres after withdrawal of life-sustaining therapy in patients with circulatory death. One hundred and sixty-eight participating centres (73%) perform a functional assessment, physical and non-physical before hospital discharge, and 71 (30%) centres have a follow-up programme within 3 months after discharge, assessing cognitive and emotional problems in 73% and 65% of the patients who were followed.
Discussion
Our results provide valuable cross-sectional information on the status of POSTCAM and implementation of guidelines in Europe and highlight the role of CACs. CACs admit more patients per year, units are better equipped and guideline recommendations3 are better implemented than non-cardiac arrest centres. Other surveys in Europe16 and the UK22 were published before 2015 ERC–ESICM guideline recommendations23 or just after and showed that treatments vary considerably between different centres and countries. The latest ERC guidelines recommendations3 were published in 2021 and 2022,24 before the survey was distributed.
Treatment of the patient who has restoration of spontaneous circulation (ROSC) after OHCA is complex and depends on the coordinated actions of diverse healthcare providers, including EMS personnel, emergency medicine physicians, cardiologists, critical care physicians, nurses, and other key personnel.3 Some studies and registries have shown an association between patients treated in high volume centres and better outcomes.25 A CAC may be defined as (i) 24/7 availability of an on-site coronary angiography laboratory; (ii) an emergency department; (iii) an intensive care unit; (iv) imaging facilities such as echocardiography, computed tomography, and magnetic resonance imaging; (v) and a protocol outlining the transfer of selected patients to cardiac arrest centres with additional resources 21 in order to achieve faster times to treatment and to be more compliant with guideline-recommended therapy.3 The implementation of CACs provides a high level of specialization and a high volume of post-resuscitation care patients, which is associated with improved patient care.26–28
Inconsistent data have been reported to support the minimum number of OHCA patients that should be treated each year by hospitals. However, treating <40 patients/year seems to be associated with an improved outcome. Our data document a median of 46.5 patients treated per centre per year, increasing to a median of 50 in CAC, showing consistency with previous data.25,29
Our results have shown poor implementation of the guidelines with respect to accessibility to 24/7 ultrasound or on-site coronary angiography, although this is better in CACs (96% and 94% of follow-up). Written protocols in the management of post-resuscitated CA may improve patient safety and guide clinicians,30 but application of protocols is poor even in CACs (overall 62% and 66% in non-CACs and CACs respectively).
The emerging role of eCPR31 use in refractory cardiac arrest needs regional organization between different systems involved (pre- and in-hospital). Establishing an eCPR programme requires considerable resources to implement effectively, and not all healthcare systems have sufficient resources.32 This aspect is highlighted in our results showing that in CACs availability is higher [CAC 90 (55%) vs. non-CAC 7 (10%), P < 0.001]. Whether or not this will change in the coming years is uncertain. Intubation and mechanical ventilation are indicated in patients with ROSC who remain comatose, in addition to avoiding hyperoxia, which is why they have not been requested in this survey.
Haemodynamic management and early angiography
Recent ERC/ESICM post-resuscitation care guidelines3 recommend avoiding hypotension and adjusted target MAP to achieve adequate urine output and normal or decreasing lactate.3 In our results, half of the participants (52%) aim for an MAP of ≥ 65 mmHg and around 11% do not have a specific target if perfusion is adequate. Variability in the answers may reflect insufficient evidence to recommend specific haemodynamic goals and should be considered on an individual patient basis. Our results are consistent with ERC/ESICM post-resuscitation care guidelines recommendations3 for noradrenaline and dobutamine as the first-line agents. Interestingly, adrenaline was used as the first vasopressor or inotrope in 14% and 17% of patients, respectively, in spite of the rising evidence suggesting a harmful effect in this setting.33 Furthermore, dobutamine use is low and did not have differences between CAC and non-CAC, in contrast with guidelines-first inotrope recommendation. Despite no benefit on outcomes with the use of IABP, this is still the most available device. The ease of use, its accessibility, and the low risk of complications make it a device of choice for many centres. However, the availability of Impella and extracorporeal membrane oxygenation (ECMO) devices is increasing (45% and 60%, respectively), in CACs. The organization of the CAC requires close collaboration with the local STEMI network. Early coronary angiography is indicated in ST elevation ECG following ROSC but not in the case of patients with stable circulation without STE in the ECG, where latest the randomized trials11,34–36 did not show benefit in this setting compared with delayed angiography during the same admission. Coronary artery disease is present in many patients with NoSTEMI, but the presence of an acute unstable lesion considered responsible for triggering cardiac arrest is less common, occurring in nearly 30% of the patients. More trials are ongoing and may provide more evidence in this field (DISCO NCT02309151), so our results presumably can change in the next few years.
Temperature control
TC is associated with improved neurological outcomes after OHCA, but the optimal target temperature has been the focus of debate. Several studies have been published in the last few years.6,37–39 Latest ERC/ESICM post-resuscitation care guidelines3 recommend for adults the remaining comatose after either OHCA or IHCA (with any initial rhythm) active prevention of fever (defined as a temperature > 37.7°C) for at least 72 h. The Targeted Hypothermia (33°C) Versus Targeted Normothermia (37.8°C) After Out-of-hospital Cardiac Arrest (TTM-2) trial did not document a lower incidence of death by 6 months than targeted normothermia.38 Whether specific subpopulations may benefit from lower temperatures remains uncertain.39,40 Data from our survey revealed that one of four CACs do not use TC in post-resuscitation care after cardiac arrest with shockable rhythms and one of three do not use TC after non-shockable rhythm cardiac arrests. Thus, even those working in CACs did not follow existing guidelines. Since the publication of the TTM-2 trial, there have been changes in temperature management in several intensive care units but TC and avoidance of fever in the first 72 h remain important and should not be abandoned.41 The ideal TC method is not well established in the literature,42,43 but methods with feedback seem to be superior to methods without temperature feedback.44 Again, the heterogeneity of clinical practice in this respect is evident from our results, with some centres having advanced technology and others continuing to use traditional means, with potentially lower efficacy and a heavier workload for the units. The variability in the method used may be determined by hospital protocols and economic implications.
Neuroprognostication
Accurate prognostication is essential so that inappropriate withdraw of life-sustaining therapy (WLST), and futile treatment is avoided in patients with severe and irreversible neurological injury. No single predictor is 100% accurate. An evaluation must begin with a neurological examination which, surprisingly, is not performed in all centres. Reasons could be related that evaluation may require transfer to another centre or department (i.e. neurology). Also, the availability of neuroprognostication tests varies even among CACs, and there is limited availability of SSEPs and NSE in many centres. ERC/ESICM post-resuscitation care guidelines recommend that neuroprognostication should always be undertaken using a multimodal approach because no single test has sufficient specificity to eliminate false positives.45 Use of a prognostication algorithm by staff with relevant expertise may enable more reliable identification of patients destined to have poor outcomes.46
Organ donation after brain death or controlled donation after circulatory death varies between countries and is likely to reflect local legal and ethical requirements. However, by collecting expertise in CACs, WLST discussions may be improved thus enhancing patient selection for potential organ donation.
Rehabilitation and follow-up
Functional assessments of physical and non-physical impairments should be conducted prior to discharge from the hospital so that early rehabilitation needs can be identified and to enable referral to rehabilitation if necessary.3 Organization varies widely between hospitals and countries. Our results show that 3 of 4 patients are assessed before discharge but 3-month follow-up is infrequent (37% of the centres). The implementation of specialized teams should be organized by the treating centre to improve patient care and to enable early detection of cognitive and emotional problems.
The importance of cardiac arrest centres
Evidence of the impact on the survival of CACs is limited but an association with improvement in survival to hospital discharge with favourable neurological outcome has been shown in some studies.47–50 These data are associated with evidence-based resuscitation care and treatments, from prehospital coordination and an in-hospital protocolized cardiorespiratory support and prognostication.51,52 The differing availability of resources throughout Europe determines that the treatment recommended by the ERC/ESICM post-resuscitation care cannot be guaranteed for all patients with OHCA and this may impact on prognosis.21
Survival after OHCA requires a strong and early application of the chain of survival.53 The fourth link, post-resuscitation care starts with good communication and coordination with EMS delivering regional systems to give the best care in the best place—the CAC. Our results show how CACs have better adherence to clinical practice guidelines than non-CACs. Cardiac arrest centres are better equipped with essential resources such as ultrasound, coronary angiography, CT scan or TC. However, working with written protocols to avoid variability, the application and duration of TC to improve neurological outcomes, and the availability and use of multimodal neuroprognostication algorithms are variable. Early rehabilitation and follow-up of survivors are very infrequent. Most of the CAC participants have the resources, but special attention should be given to the care, based on the clinical practice guidelines, that we are currently giving to resuscitated patients from OHCA.
Limitations
Selection bias of respondents limits external validity, since only a fraction of the total hospitals in Europe and some major European regions are underrepresented, and results may have been different in a larger sample. Second, our study is a cross-sectional survey and results should be considered exploratory. Outcomes were not measured, and it was not possible to correlate these with guidelines implementation. Based on our data, we cannot determine whether one treatment practise is superior to another but only describe whether treatment practices adhere to guidelines. There may be variations in adherence to guidelines due to non-clinical factors, such as financial, historical, and local expertize, and exploring these was beyond the scope of this survey. Third, data were self-reported from local registries or protocols and could be approximations in some cases.
Conclusion
This survey revealed that post-resuscitation care varies among European hospitals. Cardiac arrest centres have higher compliance with guidelines compared with respondents from non-cardiac arrest centres. The overall inconsistency in approaches and deviation from ERC/ESICM post-resuscitation care Guidelines recommendations could be a focus for improvement in future initiatives.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Funding
This document is part of the ACVC Postcam programme (European Initiative to optimize post-resuscitation care following cardiac arrest). This programme is supported by Becton Dickinson (BD) in the form of an educational grant. The programme has not been influenced in any way by its sponsor.
References
Søholm H, Kjaergaard J, Bro-Jeppesen J, Hartvig-Thomsen J, Lippert F, Køber L, et al.
Nichol G, Aufderheide TP, Eigel B, Neumar RW, Lurie KG, Bufalino VJ, et al.
Schober A, Sterz F, Laggner AN, Poppe M, Sulzgruber P, Lobmeyr E, et al.
Tranberg T, Lippert FK, Christensen EF, Stengaard C, Hjort J, Lassen JF, et al.
Abrams D, MacLaren G, Lorusso R, Price S, Yannopoulos D, Vercaemst L, et al.
Lascarrou JB, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al.
Viana-Tejedor A, Andrea-Riba R, Scardino C, Ariza-Solé A, Bañeras J, García-García C, et al.
Le May M, Osborne C, Russo J, So D, Chong AY, Dick A, et al.
Bray JE, Stub D, Bloom JE, Segan L, Mitra B, Smith K, et al.
Sandroni C, D'Arrigo S, Cacciola S, Hoedemaekers CWE, Kamps MJA, Oddo M, et al.
Scarpino M, Lolli F, Lanzo G, Carrai R, Spalletti M, Valzania F, et al.
Patterson T, Perkins GD, Joseph J, Wilson K, Van Dyck L, Robertson S, et al.
Matsuyama T, Kiyohara K, Kitamura T, Nishiyama C, Nishiuchi T, Hayashi Y, et al.
Kragholm K, Malta Hansen C, Dupre ME, Xian Y, Strauss B, Tyson C, et al.
Spaite DW, Bobrow BJ, Stolz U, Berg RA, Sanders AB, Kern KB, et al.
Olasveengen TM, Semeraro F, Ristagno G, Castren M, Handley A, Kuzovlev A, et al.
Author notes
Conflict of interest: None declared.




Comments