-
PDF
- Split View
-
Views
-
Cite
Cite
Gauravpal S Gill, Jorge Sanz Sánchez, Abhishek Thandra, Arun Kanmanthareddy, Venkata Mahesh Alla, Hector M Garcia-Garcia, Multivessel vs. culprit-vessel only percutaneous coronary interventions in acute myocardial infarction and cardiogenic shock: a systematic review and meta-analysis of prospective randomized and retrospective studies, European Heart Journal. Acute Cardiovascular Care, Volume 11, Issue 7, July 2022, Pages 558–569, https://doi.org/10.1093/ehjacc/zuac072
Close - Share Icon Share
Abstract
Studies comparing outcomes of multivessel (MV) vs. culprit-vessel (CV) only percutaneous coronary intervention (PCI) during index cardiac catheterization in patients presenting with acute myocardial infarction (MI) and cardiogenic shock (CS) have reported conflicting results. In this systematic review we aim to investigate outcomes with MV vs. CV-only revascularization strategies in patients with acute MI and CS.
PubMed, Google Scholar, CINAHL and Cochrane databases were queried for studies comparing MV vs. CV PCI in patients with acute MI and CS. Data were extracted and pooled by means of random effects model. Primary outcome was early all-cause mortality (up to 30 days), while the secondary outcomes included late all-cause mortality (mean, 11.4 months), stroke, new renal replacement therapy, reinfarction, repeat revascularization, and bleeding. Pooled odds ratio (OR), 95% confidence intervals (CIs), and number needed to harm (NNH) were calculated. A total of 16 studies enrolling 75 431 patients were included. The MV PCI was associated with higher risk of early mortality [OR 1.17, 95% CI (1.00–1.35); P = 0.04; NNH = 62], stroke [1.15 (1.03–1.29); P = 0.01; NNH = 351], and new renal replacement therapy [1.33 (1.06–1.67); P = 0.01; NNH = 61]; and with lower risk of repeat revascularization [0.61 (0.41–0.89); P = 0.01] when compared with CV PCI. No significant difference was observed in late-term mortality [1.02 (0.84–1.25); P = 0.84], risk of reinfarction [1.13 (0.94–1.35); P = 0.18], or bleeding [1.21 (0.94–1.55); P = 0.13] between groups.
Among patients with acute MI and CS, MV PCI during index cardiac catheterization was associated with higher risk of early mortality, stroke, and renal replacement therapy.
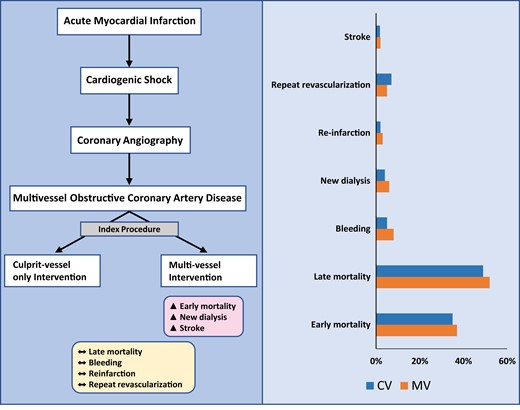
Multivessel intervention during index catheterization after acute myocardial infarction and cardiogenic shock associated with worse outcomes.
Introduction
Cardiogenic shock (CS) has been reported in up to 8% patients presenting with acute myocardial infarction (MI).1,2 During the last few decades tremendous progress has been made with development of mechanical circulatory support and newer pharmacologic therapy, while the mainstay of management continues to be early revascularization. Over the years, therapeutic advances have contributed to improved mortality outcomes in patients presenting with CS; however, the magnitude of morbidity, mortality, and financial burden from this pathology continues to be substantial with 1-year mortality rates ranging from 40 to 50%.3–6
Current European Society of Cardiology as well as American College of Cardiology/American Heart Association/Society for Coronary Angiography and Interventions guideline recommendations for coronary artery revascularization suggest percutaneous coronary intervention (PCI) of the non-culprit vessel (CV) in ST-segment elevation myocardial infarction (STEMI) as well as non-ST-segment elevation myocardial infarction (NSTEMI) complicated by CS may be harmful (Class III).7,8 Studies investigating revascularization strategies in patients presenting with acute MI and CS have predominantly included patients with STEMI.5,6,9–23 Although there has been a randomized trial that has led to guideline recommendations, investigators have continued to publish several observational studies in the recent years showing contradictory findings.17,21,22 These studies also indicate revascularization strategy for CS in the setting of acute MI was largely operator dependent.24 This is especially true for subset of patients with NSTEMI and CS, where a need for evidence is paramount. Herein, we systematically reviewed published literature comparing multivessel (MV) PCI with CV PCI during index cardiac catheterization in patients presenting with acute MI and CS. In this article, we also aim to highlight the paucity in data and limitations of current studies in guiding revascularization strategies for patients with CS, based on type of MI (NSTEMI vs. STEMI).
Methods
Search strategy and selection criteria
Randomized and observational studies comparing MV and CV PCI in patients presenting with acute MI and CS were evaluated for inclusion in this systematic review and meta-analysis. Inclusion criteria were: (i) articles published as full manuscripts in English, (ii) involved patients with acute MI and CS, and (iii) reported at least one of the outcomes (all-cause mortality, reinfarction, repeat revascularization, stroke, bleeding, and new renal replacement therapy) in acute MI and CS separately for patients undergoing primary CV and MV PCI. Exclusion criteria were: (i) duplicates of previous publications, (ii) studies which included PCI outcomes for acute MI and CS without separate data on outcomes for CV vs. MV strategies, (iii) studies that excluded patients with CS, (iv) studies which included patients with CS in acute MI without separate data on outcomes for CS patients, (v) abstracts, editorials, reviews, and commentaries, (vi) investigations from the same registry with overlap in study populations, and (vii) animal studies.
Search strategy, study selection, data extraction, and analysis were performed in accordance with The Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.25 Two authors (G.S.G. and A.T.) independently performed literature review in PubMed, Google Scholar, MEDLINE, CINHAL, and Cochrane databases from inception until 10 November 2021. Search was conducted using the keywords ‘myocardial infarction’, ‘MI’, ‘acute coronary syndrome’, ‘ST elevation myocardial infarction’, ‘STEMI’, ‘non-ST elevation myocardial infarction’, or ‘NSTEMI’ AND ‘cardiogenic shock’, or ‘CS’ AND ‘percutaneous coronary intervention’, or ‘PCI’.
Outcomes of interest
Primary endpoint was early (up to 30 days) mortality. Secondary clinical endpoints included late (mean, 11.4 months) mortality, re-infarction, repeat revascularization, stroke, bleeding, and new renal replacement therapy. Each endpoint was assessed according to the definitions reported in the original study protocols.
Data extraction
Two independent authors (G.S.G. and A.T.) performed data extraction using a standardized data extraction form. Non-relevant articles were excluded based on title and abstract. The same investigators independently extracted data on study design, measurements, patient characteristics, and outcomes, using a standardized data-extraction form. Data extraction conflicts were discussed and resolved with the senior investigator (H.M.G.G.).
Risk of bias
For the randomized clinical trial, the risk of bias has been assessed using the revised Cochrane risk of bias tool (RoB 2.0). Two investigators (J.S.S. and G.S.G.) independently assessed five domains of bias in randomized clinical trials: (i) randomization process, (ii) deviations from intended interventions, (iii) missing outcome data, (iv) measurement of the outcome, and (v) selection of the reported results. For observational studies, quality assessment was performed using the Newcastle–Ottawa scale (see Supplementary material online, Table S1).26
Statistical analysis
For all outcomes in our analyses, pooled odds ratios (ORs) with their corresponding 95% confidence intervals (CIs) were calculated using the Mantel–Haenszel random-effects model, and presented as Forest plots.27 The χ2-based Cochrane Q test was used to assess heterogeneity and I2 statistics was used for quantification. I2 index values of 25–50, 50–75, and >75% were considered low, intermediate, and high heterogeneity, respectively. Number needed to harm (NNH) was additionally computed. Publication bias was assessed using Egger’s test and visually inspected using funnel plots.
Leave one-out sensitivity analysis was performed by excluding one study at a time and repeating the analysis. All analyses were conducted using Review Manager Version 5.4.1 software (The Cochrane Collaboration, 2020, London, UK). A two-sided P-value of <0.05 was considered statistically significant.
To assess the interaction between clinical presentation (NSTEMI vs. STEMI) and treatment effect, a random-effects meta-regression analysis with the ‘empirical Bayes’ (Paule–Mandel) method to estimate the between study variance Τau2 and the Hartung–Knapp–Sidik–Jonkman adjustment was performed. The meta-regression coefficient and its corresponding P-value were reported.
Results
1937 titles and abstracts were screened, and of these, 183 relevant articles, editorials, review articles, and their references were manually reviewed for any additional studies that could qualify for inclusion. A total of 34 potentially relevant articles were selected for manuscript review, of which, 17 were excluded from final analysis (3 meta-analyses, 3 studies using same population registry, and 11 publications including review articles, letters, editorials, and investigations that did not include patients with CS; Figure 1). As there were two retrospective studies investigating outcomes from the same national database with overlapping time period, we excluded the study with smaller patient subset.20,24 In all, we included 2 publications from a prospective [The Culprit Lesion Only PCI vs. MV PCI in CS (CULPRIT-SHOCK)] trial, and 15 retrospective observational studies in our systematic review and meta-analysis.
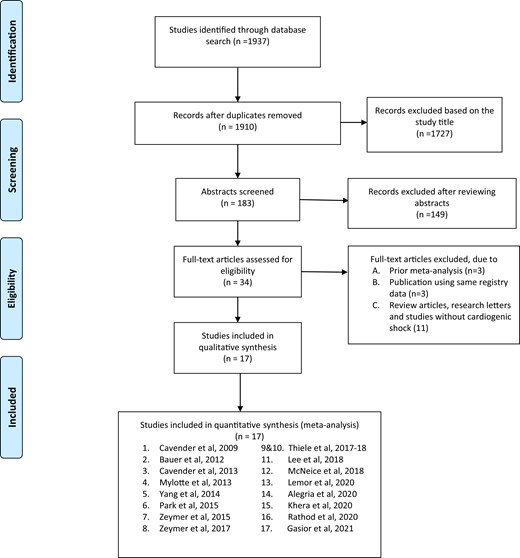
PRISMA flow chart depicting study selection for inclusion in the meta-analysis.
A total of 75 431 patients with CS and acute MI were included in the final analysis (Table 1). The study selection process as per the PRISMA guidelines is depicted in Figure 1 (see Supplementary material online, Table S2). Mean age of patients in this study was 66 years, 31% were females and 78% had STEMI (Table 2).
Study characteristics of included investigations (multivessel vs. culprit-vessel primary PCI)
| Source . | Year . | Region/country . | Study design . | Enrolment period . | Number of patients . | STEMI (%) . | Measured outcomes . | Follow-up duration . | |
|---|---|---|---|---|---|---|---|---|---|
| Culprit . | Multivessel . | ||||||||
| Cavender | 2009 | United States (NCDR), >600 centres | Retrospective | 2004–2007 | 2654 | 433 | CV: 100% | Mortality, stroke, renal failure | Until discharge |
| MV: 100% | |||||||||
| Bauer | 2012 | Europe (EHS-PCI), 176 centres | Retrospective | May 2005–Apr 2008 | 254 | 82 | CV: 87% | Mortality, non-fatal MI, non-fatal stroke, major bleeding, new renal replacement therapy, CABG | Until discharge |
| MV: 83% | |||||||||
| Cavender | 2013 | United States, single centre | Retrospective | Jan 2002–May 2010 | 156 | 43 | CV: 100% | Mortality | 2.6 years |
| MV: 100% | |||||||||
| Mylotte | 2013 | France, 5 centres | Retrospective | 1998–2010 | 103 (33 CTO) | 66 (17 CTO) | CV: 100% | Mortality, recurrent cardiac arrest, reinfarction, early urgent revascularization | 6 months |
| MV: 100% | |||||||||
| Yang | 2014 | South Korea | Retrospective | Nov 2005–Sep 2010 | 278 | 60 | CV: 100% | Mortality, major acute cardiovascular events, repeat revascularization, bleeding, stroke | 224 days |
| MV: 100% | |||||||||
| Park | 2015 | South Korea (KAMIR), 53 centres | Retrospective | Jan 2006–Dec 2012 | 386 | 124 | CV: 100% | Mortality, cardiac death, recurrent MI, bleeding, stroke, repeat revascularization and MACE | 12–24 months |
| MV: 100% | |||||||||
| Zeymer | 2015 | Germany (ALKK-PCI), 41 centres | Retrospective | Jan 2008–Dec 2011 | 562 | 173 | CV: 77% | Mortality, cardiac arrest, acute vessel closure, urgent CABG, reinfarction, stroke, bleeding, new renal replacement therapy | Until discharge |
| MV: 70% | |||||||||
| Zeymer | 2017 | Germany (IABP-SHOCK II), 41 centres | Retrospective | – | 284 | 167 | CV: 77% | Mortality, reinfarction, bleeding, new renal replacement therapy, quality of life, repeat revascularization | 1 year |
| MV: 73% | |||||||||
| Thiele | 2017 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 30 days |
| MV: 63% | |||||||||
| Thiele | 2018 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 1-year |
| MV: 63% | |||||||||
| Lee | 2018 | South Korea (KAMIR-NIH), 20 centres | Retrospective | Nov 2011–Dec 2015 | 399 | 260 | CV: 100% | Mortality, cardiac mortality, recurrent MI, repeat revascularization, new renal replacement therapy | 1 year |
| MV: 100% | |||||||||
| McNeice | 2018 | Canada (British Columbia Cardiac Registry), 5 centres | Retrospective | 2008– 2014 | 414 | 235 | CV: 76% | Mortality | 1 year |
| MV: 65% | |||||||||
| Lemor | 2020 | United States (NCSI), 57 centres | Retrospective | Jul 2016–Dec 2019 | 72 | 126 | CV: 72% | Mortality, acute kidney injury, length of stay | Until discharge |
| MV: 77% | |||||||||
| Khera | 2020 | United States (NCDR), 649 centres | Retrospective | Jul 2009–Mar 2018 | 41,883 | 22,418 | CV: 80% | Mortality, stroke, tamponade, new renal replacement therapy, bleeding, all-cause readmission, heart-failure readmission, repeat revascularization | 1 year |
| MV: 69% | |||||||||
| Rathod | 2020 | United Kingdom (BCIS PCI), 8 centres | Retrospective | Jan 2005–Jul 2015 | 561 | 497 | CV: 100% | Mortality, stroke, repeat revascularization, emergency CABG, arterial complications, bleeding | Until discharge |
| MV: 100% | |||||||||
| Alegria | 2021 | Portugal (ProACS), multicentric | Retrospective | Oct 2010–Jan 2018 | 23 | 104 | CV: 96% | Mortality, major bleeding, stroke, cardiac arrest, sustained ventricular tachycardia, atrioventricular block, reinfarction, mechanical complication | Until discharge |
| MV: 92% | |||||||||
| Gasior | 2021 | Poland (PL-ACS), multicentric | Retrospective | 2008–2019 | 883 | 1045 | CV: 72% | Mortality, recurrent MI, heart failure readmission, repeat revascularization, stroke | 1 year |
| MV: 68% | |||||||||
| Source . | Year . | Region/country . | Study design . | Enrolment period . | Number of patients . | STEMI (%) . | Measured outcomes . | Follow-up duration . | |
|---|---|---|---|---|---|---|---|---|---|
| Culprit . | Multivessel . | ||||||||
| Cavender | 2009 | United States (NCDR), >600 centres | Retrospective | 2004–2007 | 2654 | 433 | CV: 100% | Mortality, stroke, renal failure | Until discharge |
| MV: 100% | |||||||||
| Bauer | 2012 | Europe (EHS-PCI), 176 centres | Retrospective | May 2005–Apr 2008 | 254 | 82 | CV: 87% | Mortality, non-fatal MI, non-fatal stroke, major bleeding, new renal replacement therapy, CABG | Until discharge |
| MV: 83% | |||||||||
| Cavender | 2013 | United States, single centre | Retrospective | Jan 2002–May 2010 | 156 | 43 | CV: 100% | Mortality | 2.6 years |
| MV: 100% | |||||||||
| Mylotte | 2013 | France, 5 centres | Retrospective | 1998–2010 | 103 (33 CTO) | 66 (17 CTO) | CV: 100% | Mortality, recurrent cardiac arrest, reinfarction, early urgent revascularization | 6 months |
| MV: 100% | |||||||||
| Yang | 2014 | South Korea | Retrospective | Nov 2005–Sep 2010 | 278 | 60 | CV: 100% | Mortality, major acute cardiovascular events, repeat revascularization, bleeding, stroke | 224 days |
| MV: 100% | |||||||||
| Park | 2015 | South Korea (KAMIR), 53 centres | Retrospective | Jan 2006–Dec 2012 | 386 | 124 | CV: 100% | Mortality, cardiac death, recurrent MI, bleeding, stroke, repeat revascularization and MACE | 12–24 months |
| MV: 100% | |||||||||
| Zeymer | 2015 | Germany (ALKK-PCI), 41 centres | Retrospective | Jan 2008–Dec 2011 | 562 | 173 | CV: 77% | Mortality, cardiac arrest, acute vessel closure, urgent CABG, reinfarction, stroke, bleeding, new renal replacement therapy | Until discharge |
| MV: 70% | |||||||||
| Zeymer | 2017 | Germany (IABP-SHOCK II), 41 centres | Retrospective | – | 284 | 167 | CV: 77% | Mortality, reinfarction, bleeding, new renal replacement therapy, quality of life, repeat revascularization | 1 year |
| MV: 73% | |||||||||
| Thiele | 2017 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 30 days |
| MV: 63% | |||||||||
| Thiele | 2018 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 1-year |
| MV: 63% | |||||||||
| Lee | 2018 | South Korea (KAMIR-NIH), 20 centres | Retrospective | Nov 2011–Dec 2015 | 399 | 260 | CV: 100% | Mortality, cardiac mortality, recurrent MI, repeat revascularization, new renal replacement therapy | 1 year |
| MV: 100% | |||||||||
| McNeice | 2018 | Canada (British Columbia Cardiac Registry), 5 centres | Retrospective | 2008– 2014 | 414 | 235 | CV: 76% | Mortality | 1 year |
| MV: 65% | |||||||||
| Lemor | 2020 | United States (NCSI), 57 centres | Retrospective | Jul 2016–Dec 2019 | 72 | 126 | CV: 72% | Mortality, acute kidney injury, length of stay | Until discharge |
| MV: 77% | |||||||||
| Khera | 2020 | United States (NCDR), 649 centres | Retrospective | Jul 2009–Mar 2018 | 41,883 | 22,418 | CV: 80% | Mortality, stroke, tamponade, new renal replacement therapy, bleeding, all-cause readmission, heart-failure readmission, repeat revascularization | 1 year |
| MV: 69% | |||||||||
| Rathod | 2020 | United Kingdom (BCIS PCI), 8 centres | Retrospective | Jan 2005–Jul 2015 | 561 | 497 | CV: 100% | Mortality, stroke, repeat revascularization, emergency CABG, arterial complications, bleeding | Until discharge |
| MV: 100% | |||||||||
| Alegria | 2021 | Portugal (ProACS), multicentric | Retrospective | Oct 2010–Jan 2018 | 23 | 104 | CV: 96% | Mortality, major bleeding, stroke, cardiac arrest, sustained ventricular tachycardia, atrioventricular block, reinfarction, mechanical complication | Until discharge |
| MV: 92% | |||||||||
| Gasior | 2021 | Poland (PL-ACS), multicentric | Retrospective | 2008–2019 | 883 | 1045 | CV: 72% | Mortality, recurrent MI, heart failure readmission, repeat revascularization, stroke | 1 year |
| MV: 68% | |||||||||
PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; NCDR, national cardiovascular data registry; CV, culprit vessel; MV, multivessel; EHS-PCI, Euro heart survey percutaneous coronary intervention; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; KAMIR, Korea acute myocardial infarction registry; MACE, major acute cardiovascular events; ALKK-PCI, Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte percutaneous coronary intervention; IABP-SHOCK II trial, intra-aortic balloon pump in cardiogenic shock II trial; KAMIR-NIG, Korea acute myocardial infarction-National Institutes of Health; NCSI, national cardiogenic shock initiative; ProACS, Portuguese registry on acute coronary syndromes; PL-ACS, Polish registry of acute coronary syndrome.
Study characteristics of included investigations (multivessel vs. culprit-vessel primary PCI)
| Source . | Year . | Region/country . | Study design . | Enrolment period . | Number of patients . | STEMI (%) . | Measured outcomes . | Follow-up duration . | |
|---|---|---|---|---|---|---|---|---|---|
| Culprit . | Multivessel . | ||||||||
| Cavender | 2009 | United States (NCDR), >600 centres | Retrospective | 2004–2007 | 2654 | 433 | CV: 100% | Mortality, stroke, renal failure | Until discharge |
| MV: 100% | |||||||||
| Bauer | 2012 | Europe (EHS-PCI), 176 centres | Retrospective | May 2005–Apr 2008 | 254 | 82 | CV: 87% | Mortality, non-fatal MI, non-fatal stroke, major bleeding, new renal replacement therapy, CABG | Until discharge |
| MV: 83% | |||||||||
| Cavender | 2013 | United States, single centre | Retrospective | Jan 2002–May 2010 | 156 | 43 | CV: 100% | Mortality | 2.6 years |
| MV: 100% | |||||||||
| Mylotte | 2013 | France, 5 centres | Retrospective | 1998–2010 | 103 (33 CTO) | 66 (17 CTO) | CV: 100% | Mortality, recurrent cardiac arrest, reinfarction, early urgent revascularization | 6 months |
| MV: 100% | |||||||||
| Yang | 2014 | South Korea | Retrospective | Nov 2005–Sep 2010 | 278 | 60 | CV: 100% | Mortality, major acute cardiovascular events, repeat revascularization, bleeding, stroke | 224 days |
| MV: 100% | |||||||||
| Park | 2015 | South Korea (KAMIR), 53 centres | Retrospective | Jan 2006–Dec 2012 | 386 | 124 | CV: 100% | Mortality, cardiac death, recurrent MI, bleeding, stroke, repeat revascularization and MACE | 12–24 months |
| MV: 100% | |||||||||
| Zeymer | 2015 | Germany (ALKK-PCI), 41 centres | Retrospective | Jan 2008–Dec 2011 | 562 | 173 | CV: 77% | Mortality, cardiac arrest, acute vessel closure, urgent CABG, reinfarction, stroke, bleeding, new renal replacement therapy | Until discharge |
| MV: 70% | |||||||||
| Zeymer | 2017 | Germany (IABP-SHOCK II), 41 centres | Retrospective | – | 284 | 167 | CV: 77% | Mortality, reinfarction, bleeding, new renal replacement therapy, quality of life, repeat revascularization | 1 year |
| MV: 73% | |||||||||
| Thiele | 2017 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 30 days |
| MV: 63% | |||||||||
| Thiele | 2018 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 1-year |
| MV: 63% | |||||||||
| Lee | 2018 | South Korea (KAMIR-NIH), 20 centres | Retrospective | Nov 2011–Dec 2015 | 399 | 260 | CV: 100% | Mortality, cardiac mortality, recurrent MI, repeat revascularization, new renal replacement therapy | 1 year |
| MV: 100% | |||||||||
| McNeice | 2018 | Canada (British Columbia Cardiac Registry), 5 centres | Retrospective | 2008– 2014 | 414 | 235 | CV: 76% | Mortality | 1 year |
| MV: 65% | |||||||||
| Lemor | 2020 | United States (NCSI), 57 centres | Retrospective | Jul 2016–Dec 2019 | 72 | 126 | CV: 72% | Mortality, acute kidney injury, length of stay | Until discharge |
| MV: 77% | |||||||||
| Khera | 2020 | United States (NCDR), 649 centres | Retrospective | Jul 2009–Mar 2018 | 41,883 | 22,418 | CV: 80% | Mortality, stroke, tamponade, new renal replacement therapy, bleeding, all-cause readmission, heart-failure readmission, repeat revascularization | 1 year |
| MV: 69% | |||||||||
| Rathod | 2020 | United Kingdom (BCIS PCI), 8 centres | Retrospective | Jan 2005–Jul 2015 | 561 | 497 | CV: 100% | Mortality, stroke, repeat revascularization, emergency CABG, arterial complications, bleeding | Until discharge |
| MV: 100% | |||||||||
| Alegria | 2021 | Portugal (ProACS), multicentric | Retrospective | Oct 2010–Jan 2018 | 23 | 104 | CV: 96% | Mortality, major bleeding, stroke, cardiac arrest, sustained ventricular tachycardia, atrioventricular block, reinfarction, mechanical complication | Until discharge |
| MV: 92% | |||||||||
| Gasior | 2021 | Poland (PL-ACS), multicentric | Retrospective | 2008–2019 | 883 | 1045 | CV: 72% | Mortality, recurrent MI, heart failure readmission, repeat revascularization, stroke | 1 year |
| MV: 68% | |||||||||
| Source . | Year . | Region/country . | Study design . | Enrolment period . | Number of patients . | STEMI (%) . | Measured outcomes . | Follow-up duration . | |
|---|---|---|---|---|---|---|---|---|---|
| Culprit . | Multivessel . | ||||||||
| Cavender | 2009 | United States (NCDR), >600 centres | Retrospective | 2004–2007 | 2654 | 433 | CV: 100% | Mortality, stroke, renal failure | Until discharge |
| MV: 100% | |||||||||
| Bauer | 2012 | Europe (EHS-PCI), 176 centres | Retrospective | May 2005–Apr 2008 | 254 | 82 | CV: 87% | Mortality, non-fatal MI, non-fatal stroke, major bleeding, new renal replacement therapy, CABG | Until discharge |
| MV: 83% | |||||||||
| Cavender | 2013 | United States, single centre | Retrospective | Jan 2002–May 2010 | 156 | 43 | CV: 100% | Mortality | 2.6 years |
| MV: 100% | |||||||||
| Mylotte | 2013 | France, 5 centres | Retrospective | 1998–2010 | 103 (33 CTO) | 66 (17 CTO) | CV: 100% | Mortality, recurrent cardiac arrest, reinfarction, early urgent revascularization | 6 months |
| MV: 100% | |||||||||
| Yang | 2014 | South Korea | Retrospective | Nov 2005–Sep 2010 | 278 | 60 | CV: 100% | Mortality, major acute cardiovascular events, repeat revascularization, bleeding, stroke | 224 days |
| MV: 100% | |||||||||
| Park | 2015 | South Korea (KAMIR), 53 centres | Retrospective | Jan 2006–Dec 2012 | 386 | 124 | CV: 100% | Mortality, cardiac death, recurrent MI, bleeding, stroke, repeat revascularization and MACE | 12–24 months |
| MV: 100% | |||||||||
| Zeymer | 2015 | Germany (ALKK-PCI), 41 centres | Retrospective | Jan 2008–Dec 2011 | 562 | 173 | CV: 77% | Mortality, cardiac arrest, acute vessel closure, urgent CABG, reinfarction, stroke, bleeding, new renal replacement therapy | Until discharge |
| MV: 70% | |||||||||
| Zeymer | 2017 | Germany (IABP-SHOCK II), 41 centres | Retrospective | – | 284 | 167 | CV: 77% | Mortality, reinfarction, bleeding, new renal replacement therapy, quality of life, repeat revascularization | 1 year |
| MV: 73% | |||||||||
| Thiele | 2017 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 30 days |
| MV: 63% | |||||||||
| Thiele | 2018 | Europe (CULPRIT-SHOCK), multicentric | Prospective, randomized | Apr 2013–Apr 2017 | 344 | 342 | CV: 62% | Mortality, new renal replacement therapy, recurrent MI, HF readmission, staged or repeat revascularization, stroke, bleeding | 1-year |
| MV: 63% | |||||||||
| Lee | 2018 | South Korea (KAMIR-NIH), 20 centres | Retrospective | Nov 2011–Dec 2015 | 399 | 260 | CV: 100% | Mortality, cardiac mortality, recurrent MI, repeat revascularization, new renal replacement therapy | 1 year |
| MV: 100% | |||||||||
| McNeice | 2018 | Canada (British Columbia Cardiac Registry), 5 centres | Retrospective | 2008– 2014 | 414 | 235 | CV: 76% | Mortality | 1 year |
| MV: 65% | |||||||||
| Lemor | 2020 | United States (NCSI), 57 centres | Retrospective | Jul 2016–Dec 2019 | 72 | 126 | CV: 72% | Mortality, acute kidney injury, length of stay | Until discharge |
| MV: 77% | |||||||||
| Khera | 2020 | United States (NCDR), 649 centres | Retrospective | Jul 2009–Mar 2018 | 41,883 | 22,418 | CV: 80% | Mortality, stroke, tamponade, new renal replacement therapy, bleeding, all-cause readmission, heart-failure readmission, repeat revascularization | 1 year |
| MV: 69% | |||||||||
| Rathod | 2020 | United Kingdom (BCIS PCI), 8 centres | Retrospective | Jan 2005–Jul 2015 | 561 | 497 | CV: 100% | Mortality, stroke, repeat revascularization, emergency CABG, arterial complications, bleeding | Until discharge |
| MV: 100% | |||||||||
| Alegria | 2021 | Portugal (ProACS), multicentric | Retrospective | Oct 2010–Jan 2018 | 23 | 104 | CV: 96% | Mortality, major bleeding, stroke, cardiac arrest, sustained ventricular tachycardia, atrioventricular block, reinfarction, mechanical complication | Until discharge |
| MV: 92% | |||||||||
| Gasior | 2021 | Poland (PL-ACS), multicentric | Retrospective | 2008–2019 | 883 | 1045 | CV: 72% | Mortality, recurrent MI, heart failure readmission, repeat revascularization, stroke | 1 year |
| MV: 68% | |||||||||
PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; NCDR, national cardiovascular data registry; CV, culprit vessel; MV, multivessel; EHS-PCI, Euro heart survey percutaneous coronary intervention; CABG, coronary artery bypass grafting; CTO, chronic total occlusion; KAMIR, Korea acute myocardial infarction registry; MACE, major acute cardiovascular events; ALKK-PCI, Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte percutaneous coronary intervention; IABP-SHOCK II trial, intra-aortic balloon pump in cardiogenic shock II trial; KAMIR-NIG, Korea acute myocardial infarction-National Institutes of Health; NCSI, national cardiogenic shock initiative; ProACS, Portuguese registry on acute coronary syndromes; PL-ACS, Polish registry of acute coronary syndrome.
Baseline characteristics of patients from observational studies included in the meta-analysis
| Study . | Revascul-arization strategy . | N . | Age, years ± SD or years (CIs) . | Women, (%) . | HTN, (%) . | DM, (%) . | CKD, (%) . | CABG hx, (%) . | Stroke, (%) . | PVD, (%) . | HF, (%) . | STEMI, (%) . | IABP, (%) . | Impella, (%) . | DES (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavender, 2009 | Culprit | 2654 | 62 | 28 | 63 | 23 | 4 | 10 | 8 | 7 | 10 | 100 | 11 | – | – |
| MV-PCI | 453 | 60 | 29 | 60 | 25 | 4 | 5 | 8 | 6 | 13 | 100 | 16 | – | – | |
| Bauer, 2012 | Culprit | 254 | 65 ± 12 | 32 | 67 | 35 | 6 | – | 8 | 9 | 11 | 87 | 24 | – | 23 |
| MV-PCI | 82 | 67 ± 12 | 29 | 60 | 40 | 9 | – | 8 | 7 | 9 | 83 | 33 | – | 29 | |
| Cavender, 2013 | Culprit | 156 | 66 ± 13 | 38 | 79 | 31 | 10 | 11 | 10 | 15 | – | 100 | 69 | – | – |
| MV-PCI | 43 | 63 ± 14 | 28 | 72 | 35 | 19 | 12 | 7 | 14 | – | 100 | 76 | – | – | |
| Mylotte, 2013 | Culprit | 103 | 68 ± 12 | 28 | 49 | 25 | – | 5 | – | – | – | 85 | – | – | 75 |
| MV-PCI | 66 | 65 ± 12 | 34 | 53 | 26 | – | 6 | – | – | – | 82 | – | – | 60 | |
| Yang, 2014 | Culprit | 278 | 70 | 32 | 58 | 17 | – | 1 | – | – | – | 100 | – | – | 83 |
| MV-PCI | 60 | 67 | 37 | 50 | 22 | – | 0 | – | – | – | 100 | – | – | 88 | |
| Park, 2015 | Culprit | 386 | 68 (57–76) | 34 | 55 | 23 | – | – | – | – | – | 100 | 16 | – | 82 |
| MV-PCI | 124 | 66 (55–75) | 29 | 54 | 26 | – | – | – | – | – | 100 | 19 | – | 83 | |
| Zeymer, 2015 | Culprit | 562 | 70 | 29 | 78 | 35 | 40 | – | 7 | 18 | – | 77 | 41 | – | 18 |
| MV-PCI | 173 | 68 | 28 | 81 | 39 | 51 | – | 13 | 17 | – | 70 | 48 | – | 18 | |
| Zeymer, 2017 | Culprit | 284 | 68 ± 12 | 30 | 75 | 32 | – | 7 | 7 | 13 | – | 77 | 41 | – | 92 |
| MV-PCI | 167 | 69 ± 12 | 26 | 68 | 40 | – | 5 | 11 | 13 | – | 73 | 51 | – | 96 | |
| Thiele, 2017 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Thiele, 2018 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Lee, 2018 | Culprit | 399 | 67 ± 13 | 25 | 55 | 41 | 39 | – | 9 | – | – | 100 | 23 | 8 | 94 |
| MV-PCI | 260 | 66 ± 12 | 26 | 52 | 41 | 34 | – | 8 | – | – | 100 | 25 | 9 | 95 | |
| McNeice, 2018 | Culprit | 414 | – | 25 | 59 | 30 | 19 | – | 10 | 12 | – | 72 | 34 | – | – |
| MV-PCI | 235 | – | 25 | 59 | 35 | 16 | – | 10 | 10 | – | 76 | 27 | – | – | |
| Lemor, 2020 | Culprit | 72 | 63 ± 12 | 24 | – | 55 | 17 | 9 | 10 | – | 24 | 72 | – | 95 | – |
| MV-PCI | 126 | 65 ± 12 | 18 | – | 55 | 8 | 4 | 12 | – | 28 | 77 | – | 99 | – | |
| Alegria, 2020 | Culprit | 104 | 72 ± 12 | 24 | 74 | 34 | 7 | 2 | 18 | 6 | 92 | 18 | – | – | |
| MV-PCI | 23 | 63 ± 10 | 22 | 67 | 27 | 13 | 0 | 4 | 4 | 96 | 22 | – | – | ||
| Khera, 2020 | Culprit | 41883 | 67 | 32 | 71 | 32 | 33 | – | 13 | 12 | – | 80 | 48 | – | – |
| MV-PCI | 22418 | 66 | 32 | 72 | 35 | 30 | – | 13 | 13 | – | 69 | 53 | – | – | |
| Rathod, 2020 | Culprit | 561 | 68 ± 13 | 23 | 49 | 21 | 5 | – | – | 5 | – | 100 | 68 | – | 74 |
| MV-PCI | 497 | 66 ± 13 | 17 | 42 | 20 | 7 | – | – | 6 | – | 100 | 68 | – | 74 | |
| Gasior, 2021 | Culprit | 883 | 70 | 29 | 64 | 36 | – | 6 | – | – | – | 72 | – | – | 61 |
| MV-PCI | 1045 | 69 | 25 | 57 | 31 | – | 3 | – | – | – | 68 | – | – | 57 |
| Study . | Revascul-arization strategy . | N . | Age, years ± SD or years (CIs) . | Women, (%) . | HTN, (%) . | DM, (%) . | CKD, (%) . | CABG hx, (%) . | Stroke, (%) . | PVD, (%) . | HF, (%) . | STEMI, (%) . | IABP, (%) . | Impella, (%) . | DES (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavender, 2009 | Culprit | 2654 | 62 | 28 | 63 | 23 | 4 | 10 | 8 | 7 | 10 | 100 | 11 | – | – |
| MV-PCI | 453 | 60 | 29 | 60 | 25 | 4 | 5 | 8 | 6 | 13 | 100 | 16 | – | – | |
| Bauer, 2012 | Culprit | 254 | 65 ± 12 | 32 | 67 | 35 | 6 | – | 8 | 9 | 11 | 87 | 24 | – | 23 |
| MV-PCI | 82 | 67 ± 12 | 29 | 60 | 40 | 9 | – | 8 | 7 | 9 | 83 | 33 | – | 29 | |
| Cavender, 2013 | Culprit | 156 | 66 ± 13 | 38 | 79 | 31 | 10 | 11 | 10 | 15 | – | 100 | 69 | – | – |
| MV-PCI | 43 | 63 ± 14 | 28 | 72 | 35 | 19 | 12 | 7 | 14 | – | 100 | 76 | – | – | |
| Mylotte, 2013 | Culprit | 103 | 68 ± 12 | 28 | 49 | 25 | – | 5 | – | – | – | 85 | – | – | 75 |
| MV-PCI | 66 | 65 ± 12 | 34 | 53 | 26 | – | 6 | – | – | – | 82 | – | – | 60 | |
| Yang, 2014 | Culprit | 278 | 70 | 32 | 58 | 17 | – | 1 | – | – | – | 100 | – | – | 83 |
| MV-PCI | 60 | 67 | 37 | 50 | 22 | – | 0 | – | – | – | 100 | – | – | 88 | |
| Park, 2015 | Culprit | 386 | 68 (57–76) | 34 | 55 | 23 | – | – | – | – | – | 100 | 16 | – | 82 |
| MV-PCI | 124 | 66 (55–75) | 29 | 54 | 26 | – | – | – | – | – | 100 | 19 | – | 83 | |
| Zeymer, 2015 | Culprit | 562 | 70 | 29 | 78 | 35 | 40 | – | 7 | 18 | – | 77 | 41 | – | 18 |
| MV-PCI | 173 | 68 | 28 | 81 | 39 | 51 | – | 13 | 17 | – | 70 | 48 | – | 18 | |
| Zeymer, 2017 | Culprit | 284 | 68 ± 12 | 30 | 75 | 32 | – | 7 | 7 | 13 | – | 77 | 41 | – | 92 |
| MV-PCI | 167 | 69 ± 12 | 26 | 68 | 40 | – | 5 | 11 | 13 | – | 73 | 51 | – | 96 | |
| Thiele, 2017 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Thiele, 2018 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Lee, 2018 | Culprit | 399 | 67 ± 13 | 25 | 55 | 41 | 39 | – | 9 | – | – | 100 | 23 | 8 | 94 |
| MV-PCI | 260 | 66 ± 12 | 26 | 52 | 41 | 34 | – | 8 | – | – | 100 | 25 | 9 | 95 | |
| McNeice, 2018 | Culprit | 414 | – | 25 | 59 | 30 | 19 | – | 10 | 12 | – | 72 | 34 | – | – |
| MV-PCI | 235 | – | 25 | 59 | 35 | 16 | – | 10 | 10 | – | 76 | 27 | – | – | |
| Lemor, 2020 | Culprit | 72 | 63 ± 12 | 24 | – | 55 | 17 | 9 | 10 | – | 24 | 72 | – | 95 | – |
| MV-PCI | 126 | 65 ± 12 | 18 | – | 55 | 8 | 4 | 12 | – | 28 | 77 | – | 99 | – | |
| Alegria, 2020 | Culprit | 104 | 72 ± 12 | 24 | 74 | 34 | 7 | 2 | 18 | 6 | 92 | 18 | – | – | |
| MV-PCI | 23 | 63 ± 10 | 22 | 67 | 27 | 13 | 0 | 4 | 4 | 96 | 22 | – | – | ||
| Khera, 2020 | Culprit | 41883 | 67 | 32 | 71 | 32 | 33 | – | 13 | 12 | – | 80 | 48 | – | – |
| MV-PCI | 22418 | 66 | 32 | 72 | 35 | 30 | – | 13 | 13 | – | 69 | 53 | – | – | |
| Rathod, 2020 | Culprit | 561 | 68 ± 13 | 23 | 49 | 21 | 5 | – | – | 5 | – | 100 | 68 | – | 74 |
| MV-PCI | 497 | 66 ± 13 | 17 | 42 | 20 | 7 | – | – | 6 | – | 100 | 68 | – | 74 | |
| Gasior, 2021 | Culprit | 883 | 70 | 29 | 64 | 36 | – | 6 | – | – | – | 72 | – | – | 61 |
| MV-PCI | 1045 | 69 | 25 | 57 | 31 | – | 3 | – | – | – | 68 | – | – | 57 |
N, total number of patients; MV-PCI, multivessel percutaneous coronary intervention; HTN, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; CABG, coronary artery bypass graft; PVD, peripheral vascular disease; HF, heart failure; STEMI, ST-elevation myocardial infarction; IABP, intra-aortic balloon pump; DES, drug-eluting stent; MAVD, mixed aortic valve disease; MI, myocardial infarction; PVD, peripheral vascular disease; STS, Society of Thoracic Surgery; SD, standard deviation.
Baseline characteristics of patients from observational studies included in the meta-analysis
| Study . | Revascul-arization strategy . | N . | Age, years ± SD or years (CIs) . | Women, (%) . | HTN, (%) . | DM, (%) . | CKD, (%) . | CABG hx, (%) . | Stroke, (%) . | PVD, (%) . | HF, (%) . | STEMI, (%) . | IABP, (%) . | Impella, (%) . | DES (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavender, 2009 | Culprit | 2654 | 62 | 28 | 63 | 23 | 4 | 10 | 8 | 7 | 10 | 100 | 11 | – | – |
| MV-PCI | 453 | 60 | 29 | 60 | 25 | 4 | 5 | 8 | 6 | 13 | 100 | 16 | – | – | |
| Bauer, 2012 | Culprit | 254 | 65 ± 12 | 32 | 67 | 35 | 6 | – | 8 | 9 | 11 | 87 | 24 | – | 23 |
| MV-PCI | 82 | 67 ± 12 | 29 | 60 | 40 | 9 | – | 8 | 7 | 9 | 83 | 33 | – | 29 | |
| Cavender, 2013 | Culprit | 156 | 66 ± 13 | 38 | 79 | 31 | 10 | 11 | 10 | 15 | – | 100 | 69 | – | – |
| MV-PCI | 43 | 63 ± 14 | 28 | 72 | 35 | 19 | 12 | 7 | 14 | – | 100 | 76 | – | – | |
| Mylotte, 2013 | Culprit | 103 | 68 ± 12 | 28 | 49 | 25 | – | 5 | – | – | – | 85 | – | – | 75 |
| MV-PCI | 66 | 65 ± 12 | 34 | 53 | 26 | – | 6 | – | – | – | 82 | – | – | 60 | |
| Yang, 2014 | Culprit | 278 | 70 | 32 | 58 | 17 | – | 1 | – | – | – | 100 | – | – | 83 |
| MV-PCI | 60 | 67 | 37 | 50 | 22 | – | 0 | – | – | – | 100 | – | – | 88 | |
| Park, 2015 | Culprit | 386 | 68 (57–76) | 34 | 55 | 23 | – | – | – | – | – | 100 | 16 | – | 82 |
| MV-PCI | 124 | 66 (55–75) | 29 | 54 | 26 | – | – | – | – | – | 100 | 19 | – | 83 | |
| Zeymer, 2015 | Culprit | 562 | 70 | 29 | 78 | 35 | 40 | – | 7 | 18 | – | 77 | 41 | – | 18 |
| MV-PCI | 173 | 68 | 28 | 81 | 39 | 51 | – | 13 | 17 | – | 70 | 48 | – | 18 | |
| Zeymer, 2017 | Culprit | 284 | 68 ± 12 | 30 | 75 | 32 | – | 7 | 7 | 13 | – | 77 | 41 | – | 92 |
| MV-PCI | 167 | 69 ± 12 | 26 | 68 | 40 | – | 5 | 11 | 13 | – | 73 | 51 | – | 96 | |
| Thiele, 2017 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Thiele, 2018 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Lee, 2018 | Culprit | 399 | 67 ± 13 | 25 | 55 | 41 | 39 | – | 9 | – | – | 100 | 23 | 8 | 94 |
| MV-PCI | 260 | 66 ± 12 | 26 | 52 | 41 | 34 | – | 8 | – | – | 100 | 25 | 9 | 95 | |
| McNeice, 2018 | Culprit | 414 | – | 25 | 59 | 30 | 19 | – | 10 | 12 | – | 72 | 34 | – | – |
| MV-PCI | 235 | – | 25 | 59 | 35 | 16 | – | 10 | 10 | – | 76 | 27 | – | – | |
| Lemor, 2020 | Culprit | 72 | 63 ± 12 | 24 | – | 55 | 17 | 9 | 10 | – | 24 | 72 | – | 95 | – |
| MV-PCI | 126 | 65 ± 12 | 18 | – | 55 | 8 | 4 | 12 | – | 28 | 77 | – | 99 | – | |
| Alegria, 2020 | Culprit | 104 | 72 ± 12 | 24 | 74 | 34 | 7 | 2 | 18 | 6 | 92 | 18 | – | – | |
| MV-PCI | 23 | 63 ± 10 | 22 | 67 | 27 | 13 | 0 | 4 | 4 | 96 | 22 | – | – | ||
| Khera, 2020 | Culprit | 41883 | 67 | 32 | 71 | 32 | 33 | – | 13 | 12 | – | 80 | 48 | – | – |
| MV-PCI | 22418 | 66 | 32 | 72 | 35 | 30 | – | 13 | 13 | – | 69 | 53 | – | – | |
| Rathod, 2020 | Culprit | 561 | 68 ± 13 | 23 | 49 | 21 | 5 | – | – | 5 | – | 100 | 68 | – | 74 |
| MV-PCI | 497 | 66 ± 13 | 17 | 42 | 20 | 7 | – | – | 6 | – | 100 | 68 | – | 74 | |
| Gasior, 2021 | Culprit | 883 | 70 | 29 | 64 | 36 | – | 6 | – | – | – | 72 | – | – | 61 |
| MV-PCI | 1045 | 69 | 25 | 57 | 31 | – | 3 | – | – | – | 68 | – | – | 57 |
| Study . | Revascul-arization strategy . | N . | Age, years ± SD or years (CIs) . | Women, (%) . | HTN, (%) . | DM, (%) . | CKD, (%) . | CABG hx, (%) . | Stroke, (%) . | PVD, (%) . | HF, (%) . | STEMI, (%) . | IABP, (%) . | Impella, (%) . | DES (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cavender, 2009 | Culprit | 2654 | 62 | 28 | 63 | 23 | 4 | 10 | 8 | 7 | 10 | 100 | 11 | – | – |
| MV-PCI | 453 | 60 | 29 | 60 | 25 | 4 | 5 | 8 | 6 | 13 | 100 | 16 | – | – | |
| Bauer, 2012 | Culprit | 254 | 65 ± 12 | 32 | 67 | 35 | 6 | – | 8 | 9 | 11 | 87 | 24 | – | 23 |
| MV-PCI | 82 | 67 ± 12 | 29 | 60 | 40 | 9 | – | 8 | 7 | 9 | 83 | 33 | – | 29 | |
| Cavender, 2013 | Culprit | 156 | 66 ± 13 | 38 | 79 | 31 | 10 | 11 | 10 | 15 | – | 100 | 69 | – | – |
| MV-PCI | 43 | 63 ± 14 | 28 | 72 | 35 | 19 | 12 | 7 | 14 | – | 100 | 76 | – | – | |
| Mylotte, 2013 | Culprit | 103 | 68 ± 12 | 28 | 49 | 25 | – | 5 | – | – | – | 85 | – | – | 75 |
| MV-PCI | 66 | 65 ± 12 | 34 | 53 | 26 | – | 6 | – | – | – | 82 | – | – | 60 | |
| Yang, 2014 | Culprit | 278 | 70 | 32 | 58 | 17 | – | 1 | – | – | – | 100 | – | – | 83 |
| MV-PCI | 60 | 67 | 37 | 50 | 22 | – | 0 | – | – | – | 100 | – | – | 88 | |
| Park, 2015 | Culprit | 386 | 68 (57–76) | 34 | 55 | 23 | – | – | – | – | – | 100 | 16 | – | 82 |
| MV-PCI | 124 | 66 (55–75) | 29 | 54 | 26 | – | – | – | – | – | 100 | 19 | – | 83 | |
| Zeymer, 2015 | Culprit | 562 | 70 | 29 | 78 | 35 | 40 | – | 7 | 18 | – | 77 | 41 | – | 18 |
| MV-PCI | 173 | 68 | 28 | 81 | 39 | 51 | – | 13 | 17 | – | 70 | 48 | – | 18 | |
| Zeymer, 2017 | Culprit | 284 | 68 ± 12 | 30 | 75 | 32 | – | 7 | 7 | 13 | – | 77 | 41 | – | 92 |
| MV-PCI | 167 | 69 ± 12 | 26 | 68 | 40 | – | 5 | 11 | 13 | – | 73 | 51 | – | 96 | |
| Thiele, 2017 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Thiele, 2018 | Culprit | 344 | 70 | 25 | 59 | 30 | – | 6 | 9 | 13 | – | 62 | 25 | 47 | 94 |
| MV-PCI | 342 | 70 | 22 | 62 | 35 | – | 4 | 6 | 11 | – | 63 | 27 | 38 | 95 | |
| Lee, 2018 | Culprit | 399 | 67 ± 13 | 25 | 55 | 41 | 39 | – | 9 | – | – | 100 | 23 | 8 | 94 |
| MV-PCI | 260 | 66 ± 12 | 26 | 52 | 41 | 34 | – | 8 | – | – | 100 | 25 | 9 | 95 | |
| McNeice, 2018 | Culprit | 414 | – | 25 | 59 | 30 | 19 | – | 10 | 12 | – | 72 | 34 | – | – |
| MV-PCI | 235 | – | 25 | 59 | 35 | 16 | – | 10 | 10 | – | 76 | 27 | – | – | |
| Lemor, 2020 | Culprit | 72 | 63 ± 12 | 24 | – | 55 | 17 | 9 | 10 | – | 24 | 72 | – | 95 | – |
| MV-PCI | 126 | 65 ± 12 | 18 | – | 55 | 8 | 4 | 12 | – | 28 | 77 | – | 99 | – | |
| Alegria, 2020 | Culprit | 104 | 72 ± 12 | 24 | 74 | 34 | 7 | 2 | 18 | 6 | 92 | 18 | – | – | |
| MV-PCI | 23 | 63 ± 10 | 22 | 67 | 27 | 13 | 0 | 4 | 4 | 96 | 22 | – | – | ||
| Khera, 2020 | Culprit | 41883 | 67 | 32 | 71 | 32 | 33 | – | 13 | 12 | – | 80 | 48 | – | – |
| MV-PCI | 22418 | 66 | 32 | 72 | 35 | 30 | – | 13 | 13 | – | 69 | 53 | – | – | |
| Rathod, 2020 | Culprit | 561 | 68 ± 13 | 23 | 49 | 21 | 5 | – | – | 5 | – | 100 | 68 | – | 74 |
| MV-PCI | 497 | 66 ± 13 | 17 | 42 | 20 | 7 | – | – | 6 | – | 100 | 68 | – | 74 | |
| Gasior, 2021 | Culprit | 883 | 70 | 29 | 64 | 36 | – | 6 | – | – | – | 72 | – | – | 61 |
| MV-PCI | 1045 | 69 | 25 | 57 | 31 | – | 3 | – | – | – | 68 | – | – | 57 |
N, total number of patients; MV-PCI, multivessel percutaneous coronary intervention; HTN, hypertension; DM, diabetes mellitus; CKD, chronic kidney disease; CABG, coronary artery bypass graft; PVD, peripheral vascular disease; HF, heart failure; STEMI, ST-elevation myocardial infarction; IABP, intra-aortic balloon pump; DES, drug-eluting stent; MAVD, mixed aortic valve disease; MI, myocardial infarction; PVD, peripheral vascular disease; STS, Society of Thoracic Surgery; SD, standard deviation.
Follow-up duration
Two publications from the CULPRIT-SHOCK trial reported 30-day and 1-year outcomes, respectively.5,6 All 16 investigations reported early mortality (in-hospital or 30 days), while 10 investigations reported late mortality (6-month to 1-year follow-up, mean 11.4 months).9–23 Follow-up durations for individual studies are shown in Table 1.
Clinical outcomes and sensitivity analyses
Primary outcome
The pooled early mortality rate was 36% (37% in MV PCI and 35% in CV PCI) patients, while late mortality occurred in 50% (52% in MV PCI and 49% in CV PCI) patients. Patients undergoing MV PCI when compared with those undergoing CV PCI showed significantly higher risk of early mortality [OR 1.17, 95% CI (1.00–1.35); P = 0.04; I2 = 75%], which corresponds to an NNH value of 62 (Figure 2). On exclusion sensitivity analysis excluding one study at a time, the results remained consistent, and the heterogeneity remained moderate for early (lowest I2 = 69% on exclusion of study by Khera et al.) mortality outcome.
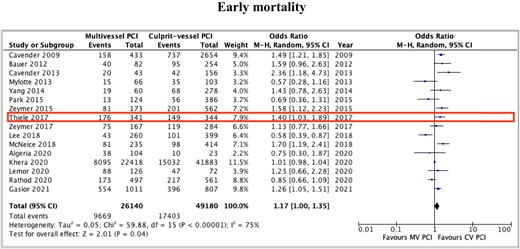
Early mortality outcomes with multivessel vs. culprit-vessel only percutaneous coronary intervention during index revascularization procedure among patients with acute myocardial infarction and cardiogenic shock. Prospective randomized study is highlighted.
Secondary outcomes
Late (mean follow-up 11.4 months) mortality was similar between the MV and CV PCI groups [1.02 (0.84–1.25); P = 0.84; Figure 3A]. There was high heterogeneity among included studies, with I2 = 76%. Results remained consistent on exclusion sensitivity analysis, while the heterogeneity decreased to moderate at best (I2 = 67%) on exclusion of study by Lee et al. We additionally conducted meta-regression analysis to assess the influence of type of acute MI on the outcome by revascularization strategy. While the type of acute MI (STEMI vs. NSTEMI) did not have a significant impact on the early mortality, in the case of late mortality, it was noted that MV PCI during index cardiac catheterization was associated with lower late-term mortality as the proportion of patients presenting with STEMI increased (Figure 4).
Secondary outcomes: late-term mortality (A), bleeding (B), new renal replacement therapy (C), re-infarction (D), repeat revascularization (E), and stroke (F) with multivessel vs. culprit-vessel only percutaneous coronary intervention during index revascularization procedure among patients with acute myocardial infarction and cardiogenic shock. Prospective randomized study is highlighted.
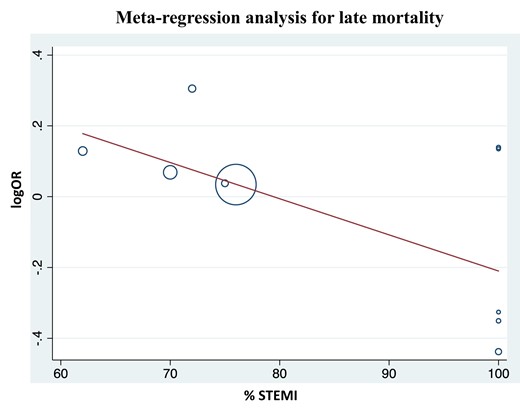
Meta-regression showing percentage of patients presenting with ST-segment elevation myocardial infarction modified the effect of multivessel percutaneous coronary intervention on the risk of late-term mortality.
There was no difference in risk of bleeding events between the MV PCI and the CV PCI groups [1.21 (0.94–1.55); P = 0.13; Figure 3B]. There was, however, moderate heterogeneity among included studies with I2 = 50%. On exclusion sensitivity analysis, it was noted that the independent exclusion of studies by Rathod et al. and by Zeymer et al., via jack-knife approach made the between group differences significant, with higher odds of bleeding with MV PCI during index catheterization. The heterogeneity was reduced to mild with I2 = 27 and 47%, respectively, on exclusion of these studies. On exclusion of study by Khera et al., the heterogeneity was significantly reduced with I2 = 0%, although results remained consistent without significant difference in bleeding between the two groups.
There was higher risk of new renal replacement therapy associated with MV PCI during index catheterization [1.33 (1.06–1.67); P = 0.01; NNH = 61; Figure 3C]. There was low heterogeneity with I2 = 34%. Results remained consistent on leave one-out exclusionary sensitivity analyses.
The odds of reinfarction were similar [1.13 (0.94–1.35); P = 0.18] between the two groups (Figure 3D). There was no significant heterogeneity with I2 = 0%. These results remained consistent on exclusion sensitivity analysis. In contrast, odds of repeat revascularization were lower with MV primary PCI [0.61 (0.41–0.89); P = 0.01; NNH = 47; Figure 3E], though there was high heterogeneity associated with I2 = 79%. The results remained consistent with exclusionary sensitivity analysis, although heterogeneity remained moderate at best upon exclusion of the randomized trial by Thiele et al. with I2 = 68%.
Patients who underwent MV PCI were associated with higher odds of stroke events [1.15 (1.03–1.29); P = 0.01; NNH = 351; Figure 3F]. There was no heterogeneity among studies included to estimate difference in stroke outcomes with I2 = 0%. On exclusion sensitivity analysis using jack-knife approach, the results remained consistent in direction and magnitude, although P-value was non-significant when the largest CathPCI-based study by Khera et al. was excluded.
Funnel-plot distributions of the pre-specified outcomes as well as Egger’s tests indicated absence of publication bias and small study effect for all the outcomes (see Supplementary material online, Figure S1).
Discussion
The major findings of this analysis are (i) MV PCI during index intervention among patients with acute MI and CS is associated with higher risk of early mortality when compared with CV PCI, (ii) MV PCI is associated with higher risk of stroke and new renal replacement therapy, (iii) MV PCI is associated with lower risk of repeat revascularization when compared with CV PCI, and (iv) There is no observed difference in risk of bleeding and reinfarction between the two groups.
Mortality outcomes have been similar across randomized trials when comparing MV and CV PCI among patients with STEMI. A meta-analysis of these randomized studies showed there is no difference in all-cause mortality, major bleeding, stroke, and contrast induced nephropathy, while the odds of reinfarction, repeat revascularization, and cardiovascular mortality are lower with MV PCI.28 Among patients with acute MI that is complicated with CS, the decision to adopt a CV vs. MV PCI strategy during index procedure is, however, more complicated. Multivessel disease is common in patients with acute MI and CS, and much like STEMI patients without shock, having MV disease is associated with a poor prognosis.4,29,30 Current European and American Society guidelines recommend against routine revascularization of non-culprit lesions in acute MI complicated by CS during primary PCI.7,8 Most evidence in this population originates from retrospective studies which have inherent limitations.31 In this analysis, we have computed outcomes from 16 studies, which include one prospective and 15 retrospective investigations. All studies reported outcomes by PCI strategy, and defined MV and CV PCI based on index cardiac catheterization procedure. Not surprisingly, study population characteristics differed across these studies resulting in significant heterogeneity. For example, study by Mylotte et al.12 exclusively included patients who had a cardiac arrest in addition to acute MI and CS. The most important difference among studies was the proportion of NSTEMI patients included (mean 22%, range 0–38%). This population is especially under-represented across studies, and evidence guiding revascularization strategy in these patients has been lagging.
In accordance with the current guidelines, our study demonstrates MV PCI strategy is associated with more harm than benefit, as exhibited by higher odds of early mortality, stroke, and new renal replacement therapy. Although we noted intermediate to high heterogeneity, exclusion sensitivity analyses showed consistent directionality and strength, while heterogeneity lowered to intermediate at best. Furthermore, these results echo findings from the randomized trial included in this analysis although several subsequent observational studies that have since been published show contradicting results. There are several possible explanations for these findings. First, the decision to perform CV PCI vs. MV PCI was operator dependent in most retrospective studies, and it may be possible that MV PCI was attempted in patients with more severe disease, thus leading to worse outcomes. Second, since CV PCI and MV PCI were defined based on index procedure, late mortality outcomes may be similar due to staged procedures performed during the follow-up period. Third, studies did not consistently report prevalence and interventions of chronic total occlusions, which may carry a higher rate of complications, contrast exposure, and repeat revascularization. Fourth, the assessment of non-culprit lesions for physiologic significance was not similar across studies, and non-standardized even within the same study, contributing to significant heterogeneity. Fifth, there was a significant difference in utilization rates of mechanical circulatory support devices across studies, including a difference in the proportion of intra-aortic balloon pump and Impella used. Since utilization of these devices have been associated with differences in short- and long-term outcomes, it is quite possible that the effects of differential use of these devices may have led to heterogeneity.32 Lastly, in most registry-based studies, late-term mortality was computed from insurance linked data, where in-hospital mortality events were not included and late mortality solely analysed events among patients discharged alive.
Meta-regression analysis performed to investigate differential outcomes between STEMI and NSTEMI populations showed MV PCI is associated with lower late mortality as proportion of STEMI patients increased. This is an intriguing finding since the culprit lesion is evident in STEMI patients; however, among those presenting with NSTEMI, culprit lesion was often defined as the tightest stenosis, which may not always be indicative of ruptured or vulnerable plaque site.33 One plausible explanation for this finding is that patients with STEMI have a much more pro-inflammatory milieu compared with NSTEMI which increases vulnerability of non-culprit lesions, revascularizing such lesions in STEMI may lead to greater benefit compared with NSTEMI.
Results from our analysis indicate higher rates of stroke and new renal replacement therapy, but lower rate of repeat revascularization rates among patients undergoing MV PCI during the index procedure. Heterogeneity was low for stroke and new renal replacement, and high for repeat revascularization. The heterogeneity may be explained by the varying rates of staged PCI subsequent to the index procedure, variability in operator, and hospital practices in choosing to perform CV or MV PCI, variable rates of physiologic assessment of non-culprit lesions and differences in stent types. The higher stroke rates and new renal replacement therapy can potentially be explained by the increased procedure duration, greater catheter manipulation and exchanges, and higher contrast exposure with MV PCI.4–5
Finally, our results demonstrate that the incidence of bleeding events and reinfarction were similar to MV PCI and CV PCI during index intervention. Although there was low heterogeneity for reinfarction, the bleeding outcome showed low to intermediate heterogeneity. On exclusion sensitivity analysis, the results remained consistent; however, heterogeneity was reduced to low on independent exclusion of studies by Rathod et al. (highest intra-aortic balloon pump use) and Khera et al. (second highest intra-aortic balloon pump use). This may be explained by the difference in definition of bleeding events across the studies. While some analyses included only major bleeding events, others reported all bleeding events.
The findings from our study have important clinical implications. Multivessel disease in patients presenting with acute MI and CS is associated with higher rates of adverse outcomes. Currently, revascularization strategies are largely operator dependent. Clearly, there is limited evidence available to guide treatment of MVD in patients with NSTEMI and CS, and it is important to investigate this population separately from those with STEMI. Future studies should standardize methods of defining CV, use of physiologic assessment in MV disease, and accounting for staged procedures. This notion is supported by a propensity matched analysis where MV PCI was actually associated with lower early mortality contrary to this meta-analysis.24
Limitations and strengths
Our study has several limitations. First, among the studies included in this meta-analysis, 15 studies have an observational design, and their limitations are inherited by our analysis with residual bias being likely. Second, given the lack of patient-level data in our study, the impact of underlying risk factors such as patient demographic, stent type, door to balloon time, use of mechanical support, cardiac arrest, disease anatomy including proportion of chronic total occlusions, timing and successful revascularization with staged procedures, etc. could not be assessed using multivariate analyses or propensity-score matching. Third, MV revascularization may not always mean complete revascularization, and this may add to high degree of heterogeneity based on degree of revascularization achieved. Importantly, this meta-analysis shows significant heterogeneity. We performed several sensitivity analyses to document the consistency of our results despite the aforementioned limitations. We employed jack-knife approach and excluded individual studies to investigate outcomes and recalculate I2. Despite these limitations, our analysis provides valuable insights into the differential outcomes of MV PCI and CV PCI in patients with acute MI and CS, which will need to be confirmed in large future prospective studies.
Conclusions
The MV PCI during index revascularization procedure in patients with acute MI and CS is associated with higher risk of early mortality. The MV PCI is also associated with higher stroke and new renal replacement therapy rates, and lower revascularization rates. Future studies are needed to investigate optimum revascularization strategy by type of MI (NSTEMI and STEMI) among patients presenting with CS.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest: None declared.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.






Comments